Dynamic expression of Lgr5, a Wnt target gene, in the developing and mature mouse cochlea
- PMID: 21472479
- PMCID: PMC3123443
- DOI: 10.1007/s10162-011-0267-2
Dynamic expression of Lgr5, a Wnt target gene, in the developing and mature mouse cochlea
Abstract
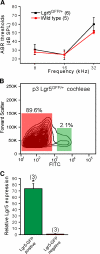
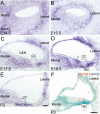
The Wnt signaling pathway is a recurring theme in tissue development and homeostasis. Its specific roles during inner ear development are just emerging, but few studies have characterized Wnt target genes. Lgr5, a member of the G protein-coupled receptor family, is a Wnt target in the gastrointestinal and integumentary systems. Although its function is unknown, its deficiency leads to perinatal lethality due to gastrointestinal distension. In this study, we used a knock-in reporter mouse to examine the spatiotemporal expression of Lgr5 in the cochlear duct during embryonic and postnatal periods. In the embryonic day 15.5 (E15.5) cochlear duct, Lgr5-EGFP is expressed in the floor epithelium and overlapped with the prosensory markers Sox2, Jagged1, and p27(Kip1). Nascent hair cells and supporting cells in the apical turn of the E18.5 cochlear duct express Lgr5-EGFP, which becomes downregulated in hair cells and subsets of supporting cells in more mature stages. In situ hybridization experiments validated the reporter expression, which gradually decreases until the second postnatal week. Only the third row of Deiters' cells expresses Lgr5-EGFP in the mature organ of Corti. Normal cochlear development was observed in Lgr5(EGFP/EGFP) and Lgr5(EGFP/+) mice, which exhibited normal auditory thresholds. The expression pattern of Lgr5 contrasts with another Wnt target gene, Axin2, a feedback inhibitor of the Wnt pathway. Robust Axin2 expression was found in cells surrounding the embryonic cochlear duct and becomes restricted to tympanic border cells below the basilar membrane in the postnatal cochlea. Both Lgr5 and Axin2 act as Wnt targets in the cochlea because purified Wnt3a promoted and Wnt antagonist suppressed their expression. Their differential expression among cell populations highlights the dynamic but complex distribution of Wnt-activated cells in and around the embryonic and postnatal cochlea.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

