Addition of pamidronate to chemotherapy for the treatment of osteosarcoma
- PMID: 21472721
- PMCID: PMC3059356
- DOI: 10.1002/cncr.25744
Addition of pamidronate to chemotherapy for the treatment of osteosarcoma
Abstract
Background: This study evaluated the safety and feasibility of the addition of pamidronate to chemotherapy for treatment of osteosarcoma.
Methods: The authors treated 40 patients with osteosarcoma with cisplatin, doxorubicin, and methotrexate with the addition of pamidronate 2 mg/kg/dose (max dose 90 mg) monthly for 12 doses. Survival, event-free survival (EFS), and durability of orthopedic reconstruction were evaluated.
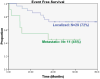
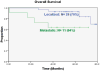
Results: For patients with localized disease, event-free survival (EFS) at 5 years was 72% and overall survival 93%. For patients with metastatic disease, EFS at 5 years was 45% and overall survival 64%. Toxicity was similar to patients treated with chemotherapy alone. Thirteen of 14 uncemented implants demonstrated successful osteointegration. Among allograft reconstructions, there were 2 graft failures, 4 delayed unions, and 6 successful grafts. Overall, 5 of 33 reconstructions failed. There were no stress fractures or growth disturbances.
Conclusions: Pamidronate can be safely incorporated with chemotherapy for the treatment of osteosarcoma. It does not impair the efficacy of chemotherapy. Pamidronate may improve the durability of limb reconstruction.
Copyright © 2010 American Cancer Society.
Figures
References
-
- Meyers PA, et al. Chemotherapy for nonmetastatic osteogenic sarcoma: the Memorial Sloan-Kettering experience. J Clin Oncol. 1992;10(1):5–15. - PubMed
-
- Link MP, et al. The effect of adjuvant chemotherapy on relapse-free survival in patients with osteosarcoma of the extremity. N Engl J Med. 1986;314(25):1600–6. - PubMed
-
- Baum ES, et al. Phase II study of cis-dichlorodiammineplatinum(II) in childhood osteosarcoma: Children’s Cancer Study Group Report. Cancer Treat Rep. 1979;63(9–10):1621–7. - PubMed
-
- Cortes EP, Holland JF, Wang JJ, Sinks LF. Doxorubicin in disseminated osteosarcoma. JAMA. 1972;3144(25):1132–1138. - PubMed
-
- Jaffe N, et al. Favorable response of metastatic osteogenic sarcoma to pulse high-dose methotrexate with citrovorum rescue and radiation therapy. Cancer. 1973;31(6):1367–73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical