Nucleosides accelerate inflammatory osteolysis, acting as distinct innate immune activators
- PMID: 21472777
- PMCID: PMC4636195
- DOI: 10.1002/jbmr.400
Nucleosides accelerate inflammatory osteolysis, acting as distinct innate immune activators
Abstract
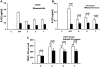
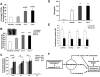
The innate immune system and its components play an important role in the pathogenesis of inflammatory bone destruction. Blockade of inflammatory cytokines does not completely arrest bone erosion, suggesting that other mediators also may be involved in osteolysis. Previously we showed that nucleosides promote osteoclastogenesis and bone-resorption activity in the presence of receptor activator for nuclear factor κB ligand (RANKL) in vitro. The studies described here further demonstrate that selected nucleosides and nucleoside analogues accelerate bone destruction in mice immunized with collagen II alone (CII) but also further enhance bone erosion in mice immunized by collagen II plus complete Freund's adjuvant (CII + CFA). Abundant osteoclasts are accumulated in destructive joints. These data indicate that nucleosides act as innate immune activators distinct from CFA, synergistically accelerating osteoclast formation and inflammatory osteolysis. The potential roles of the surface triggering receptor expressed on myeloid cells (TREM) and the intracellular inflammasome in nucleoside-enhanced osteoclastogenesis have been studied. These observations provide new insight into the pathogenesis and underlying mechanism of bone destruction in inflammatory autoimmune osteoarthritis.
Copyright © 2011 American Society for Bone and Mineral Research.
Conflict of interest statement
Figures




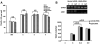
Similar articles
-
Blockade of XCL1/Lymphotactin Ameliorates Severity of Periprosthetic Osteolysis Triggered by Polyethylene-Particles.Front Immunol. 2020 Aug 4;11:1720. doi: 10.3389/fimmu.2020.01720. eCollection 2020. Front Immunol. 2020. PMID: 32849609 Free PMC article.
-
Inhibition of inflammatory bone erosion by constitutively active STAT-6 through blockade of JNK and NF-kappaB activation.Arthritis Rheum. 2005 Sep;52(9):2719-29. doi: 10.1002/art.21286. Arthritis Rheum. 2005. PMID: 16142755
-
Puerarin inhibits titanium particle-induced osteolysis and RANKL-induced osteoclastogenesis via suppression of the NF-κB signaling pathway.J Cell Mol Med. 2020 Oct;24(20):11972-11983. doi: 10.1111/jcmm.15821. Epub 2020 Sep 7. J Cell Mol Med. 2020. PMID: 32896108 Free PMC article.
-
Mechanisms modulating inflammatory osteolysis: a review with insights into therapeutic targets.Pathol Res Pract. 2008;204(10):695-706. doi: 10.1016/j.prp.2008.07.002. Epub 2008 Aug 30. Pathol Res Pract. 2008. PMID: 18757139 Free PMC article. Review.
-
Inflammasomes in Bone Diseases.Exp Suppl. 2018;108:269-279. doi: 10.1007/978-3-319-89390-7_11. Exp Suppl. 2018. PMID: 30536175 Review.
Cited by
-
The Role of NLRP3 Inflammasome Activities in Bone Diseases and Vascular Calcification.Inflammation. 2021 Apr;44(2):434-449. doi: 10.1007/s10753-020-01357-z. Epub 2020 Nov 20. Inflammation. 2021. PMID: 33215255 Free PMC article. Review.
-
Regulatory Role of Apoptotic and Inflammasome Related Proteins and Their Possible Functional Aspect in Thiram Associated Tibial Dyschondroplasia of Poultry.Animals (Basel). 2022 Aug 10;12(16):2028. doi: 10.3390/ani12162028. Animals (Basel). 2022. PMID: 36009620 Free PMC article. Review.
-
T-cell exhaustion in allograft rejection and tolerance.Curr Opin Organ Transplant. 2015 Feb;20(1):37-42. doi: 10.1097/MOT.0000000000000153. Curr Opin Organ Transplant. 2015. PMID: 25563990 Free PMC article. Review.
References
-
- McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 2007;7:429–442. - PubMed
-
- Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol Cell. 2002;10:417–426. - PubMed
-
- Deane JA, Bolland S. Nucleic acid-sensing TLRs as modifiers of autoimmunity. J Immunol. 2006;177:6573–6578. - PubMed

