Fibroblast growth factor 23 regulates renal 1,25-dihydroxyvitamin D and phosphate metabolism via the MAP kinase signaling pathway in Hyp mice
- PMID: 21472778
- PMCID: PMC4409871
- DOI: 10.1002/jbmr.401
Fibroblast growth factor 23 regulates renal 1,25-dihydroxyvitamin D and phosphate metabolism via the MAP kinase signaling pathway in Hyp mice
Abstract
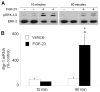
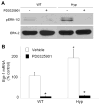
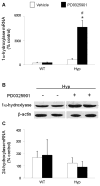
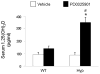
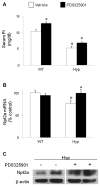
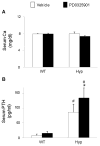
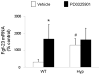
In X-linked hypophosphatemia (XLH) and in its murine homologue, the Hyp mouse, increased circulating concentrations of fibroblast growth factor 23 (FGF-23) are critical to the pathogenesis of disordered metabolism of phosphate (P(i)) and 1,25-dihydroxyvitamin D [1,25(OH)(2)D]. In this study, we hypothesized that in Hyp mice, FGF-23-mediated suppression of renal 1,25(OH)(2)D production and P(i) reabsorption depends on activation of mitogen-activated protein kinase (MAPK) signaling. Wild-type and Hyp mice were administered either vehicle or the MEK inhibitor PD0325901 (12.5 mg/kg) orally daily for 4 days. At baseline, the renal abundance of early growth response 1 (egr1) mRNA was approximately 2-fold greater in Hyp mice than in wild-type mice. Treatment with PD0325901 greatly suppressed egr1 mRNA abundance in both wild-type and Hyp mice. In Hyp mice, PD0325901 induced an 8-fold increase in renal 1α-hydroxylase mRNA expression and a 4-fold increase in serum 1,25(OH)(2)D concentrations compared with vehicle-treated Hyp mice. Serum P(i) levels in Hyp mice increased significantly after treatment with PD0325901, and the increase was associated with increased renal Npt2a mRNA abundance and brush-border membrane Npt2a protein expression. These findings provide evidence that in Hyp mice, MAPK signaling is constitutively activated in the kidney and support the hypothesis that the FGF-23-mediated suppression of renal 1,25(OH)(2)D production and P(i) reabsorption depends on activation of MAPK signaling via MEK/ERK1/2. These findings demonstrate the physiologic importance of MAPK signaling in the actions of FGF-23 in regulating renal 1,25(OH)(2)D and P(i) metabolism.
Copyright © 2011 American Society for Bone and Mineral Research.
Conflict of interest statement
All the authors state that they have no conflicts of interest.
Figures
References
-
- Yamazaki Y, Okazaki R, Shibata M, et al. Increased circulatory level of biologically active full-length FGF-23 in patients with hypophosphatemic rickets/osteomalacia. J Clin Endocrinol Metab. 2002;87:4957–4960. - PubMed
-
- Jonsson KB, Zahradnik R, Larsson T, et al. Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N Engl J Med. 2003;348:1656–1663. - PubMed
-
- Imel EA, Hui SL, Econs MJ. FGF-23 concentrations vary with disease status in autosomal dominant hypophosphatemic rickets. J Bone Miner Res. 2007;22:520–526. - PubMed
-
- Anonymous. A gene (PEX) with homologies with endopeptidases is mutated in patients with X-linked hypophosphatemic rickets. The HYP Consortium. Nat Genet. 1995;11:130–136. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

