Binding of the cSH3 domain of Grb2 adaptor to two distinct RXXK motifs within Gab1 docker employs differential mechanisms
- PMID: 21472810
- PMCID: PMC3116947
- DOI: 10.1002/jmr.1080
Binding of the cSH3 domain of Grb2 adaptor to two distinct RXXK motifs within Gab1 docker employs differential mechanisms
Abstract
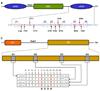
A ubiquitous component of cellular signaling machinery, Gab1 docker plays a pivotal role in routing extracellular information in the form of growth factors and cytokines to downstream targets such as transcription factors within the nucleus. Here, using isothermal titration calorimetry (ITC) in combination with macromolecular modeling (MM), we show that although Gab1 contains four distinct RXXK motifs, designated G1, G2, G3, and G4, only G1 and G2 motifs bind to the cSH3 domain of Grb2 adaptor and do so with distinct mechanisms. Thus, while the G1 motif strictly requires the PPRPPKP consensus sequence for high-affinity binding to the cSH3 domain, the G2 motif displays preference for the PXVXRXLKPXR consensus. Such sequential differences in the binding of G1 and G2 motifs arise from their ability to adopt distinct polyproline type II (PPII)- and 3(10) -helical conformations upon binding to the cSH3 domain, respectively. Collectively, our study provides detailed biophysical insights into a key protein-protein interaction involved in a diverse array of signaling cascades central to health and disease.
Copyright © 2010 John Wiley & Sons, Ltd.
Figures




References
-
- Alkarain A, Jordan R, Slingerland J. p27 deregulation in breast cancer: prognostic significance and implications for therapy. J Mammary Gland Biol Neoplasia. 2004;9:67–80. - PubMed
-
- Araki T, Nawa H, Neel BG. Tyrosyl phosphorylation of Shp2 is required for normal ERK activation in response to some, but not all, growth factors. J Biol Chem. 2003;278:41677–41684. - PubMed
-
- Bennett HL, Brummer T, Jeanes A, Yap AS, Daly RJ. Gab2 and Src co-operate in human mammary epithelial cells to promote growth factor independence and disruption of acinar morphogenesis. Oncogene. 2008;27:2693–2704. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

