Vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix: a multicenter, randomized, double-blind, placebo-controlled trial
- PMID: 21472815
- PMCID: PMC3482512
- DOI: 10.1002/uog.9017
Vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix: a multicenter, randomized, double-blind, placebo-controlled trial
Abstract
Objectives: Women with a sonographic short cervix in the mid-trimester are at increased risk for preterm delivery. This study was undertaken to determine the efficacy and safety of using micronized vaginal progesterone gel to reduce the risk of preterm birth and associated neonatal complications in women with a sonographic short cervix.
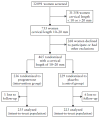
Methods: This was a multicenter, randomized, double-blind, placebo-controlled trial that enrolled asymptomatic women with a singleton pregnancy and a sonographic short cervix (10-20 mm) at 19 + 0 to 23 + 6 weeks of gestation. Women were allocated randomly to receive vaginal progesterone gel or placebo daily starting from 20 to 23 + 6 weeks until 36 + 6 weeks, rupture of membranes or delivery, whichever occurred first. Randomization sequence was stratified by center and history of a previous preterm birth. The primary endpoint was preterm birth before 33 weeks of gestation. Analysis was by intention to treat.
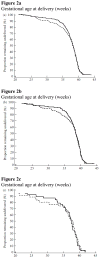
Results: Of 465 women randomized, seven were lost to follow-up and 458 (vaginal progesterone gel, n=235; placebo, n=223) were included in the analysis. Women allocated to receive vaginal progesterone had a lower rate of preterm birth before 33 weeks than did those allocated to placebo (8.9% (n=21) vs 16.1% (n=36); relative risk (RR), 0.55; 95% CI, 0.33-0.92; P=0.02). The effect remained significant after adjustment for covariables (adjusted RR, 0.52; 95% CI, 0.31-0.91; P=0.02). Vaginal progesterone was also associated with a significant reduction in the rate of preterm birth before 28 weeks (5.1% vs 10.3%; RR, 0.50; 95% CI, 0.25-0.97; P=0.04) and 35 weeks (14.5% vs 23.3%; RR, 0.62; 95% CI, 0.42-0.92; P=0.02), respiratory distress syndrome (3.0% vs 7.6%; RR, 0.39; 95% CI, 0.17-0.92; P=0.03), any neonatal morbidity or mortality event (7.7% vs 13.5%; RR, 0.57; 95% CI, 0.33-0.99; P=0.04) and birth weight < 1500 g (6.4% (15/234) vs 13.6% (30/220); RR, 0.47; 95% CI, 0.26-0.85; P=0.01). There were no differences in the incidence of treatment-related adverse events between the groups.
Conclusions: The administration of vaginal progesterone gel to women with a sonographic short cervix in the mid-trimester is associated with a 45% reduction in the rate of preterm birth before 33 weeks of gestation and with improved neonatal outcome.
Copyright © 2011 ISUOG. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
S.S.H., R.R., M.T.G., A.C.A., W.K. and L.S. have no financial interest. Author-investigators D.V., S.F., J.B., M.K., J.V., Y.T., P.S.-P., P.S., A.D., V.P., J.O.’B., V.A., O.Y., B.D., H.S., L.M. and D.M. conducted this study with the support of grants awarded by Columbia Laboratories, Inc. for the specific purpose of conducting this trial. The terms and conditions for the awarding of the grants were consistent with those which are customary for this type of industry-sponsored trial and all payments were independent of the outcome of the trial. In addition, J.K.B. and J.O.’B. have also received consulting fees and travel expenses related to Preterm Birth Advisory Committee meetings related to the project. J.O.’B. is an inventor on a patent for the use of progesterone in the prevention of preterm birth. J.A.P. received remuneration as a statistical consultant to Columbia Laboratories, Inc. G.W.C. is an employee of Columbia Laboratories, Inc.
Figures
Comment in
-
Universal cervical-length screening and vaginal progesterone prevents early preterm births, reduces neonatal morbidity and is cost saving: doing nothing is no longer an option.Ultrasound Obstet Gynecol. 2011 Jul;38(1):1-9. doi: 10.1002/uog.9073. Ultrasound Obstet Gynecol. 2011. PMID: 21713990 No abstract available.
-
What is new in preterm birth prevention? Important recent articles.Obstet Gynecol. 2013 Aug;122(2 Pt 1):390-392. doi: 10.1097/AOG.0b013e31829e41fc. Obstet Gynecol. 2013. PMID: 23969810
-
Progesterone for preterm labour.BJOG. 2016 Nov;123(12):2000. doi: 10.1111/1471-0528.13976. Epub 2016 Mar 30. BJOG. 2016. PMID: 27028883 No abstract available.
-
The unmeasured cost of preterm labor screening programs.Acta Obstet Gynecol Scand. 2017 Apr;96(4):393-394. doi: 10.1111/aogs.13125. Acta Obstet Gynecol Scand. 2017. PMID: 28345227 No abstract available.
-
Screening and prevention of preterm birth: what is a clinician to do?Acta Obstet Gynecol Scand. 2017 Aug;96(8):905-906. doi: 10.1111/aogs.13142. Acta Obstet Gynecol Scand. 2017. PMID: 28726322 No abstract available.
References
-
- Behrman RE, Butler AS, editors. Preterm Birth Causes, Consequences, and Prevention. Institute of Medicine of the National Academies. The National Academies Press; Washington D.C: 2007. Committee on Understanding Premature Birth and Assuring Healthy Outcomes, Board on Health Sciences Policy. - PubMed
-
- Bernstine RL, Lee SH, Crawford WL, Shimek MP. Sonographic evaluation of the incompetent cervix. J Clin Ultrasound. 1981;9:417–420. - PubMed
-
- Feingold M, Brook I, Zakut H. Detection of cervical incompetence by ultrasound. Acta Obstet Gynecol Scand. 1984;63:407–410. - PubMed
-
- Michaels WH, Montgomery C, Karo J, Temple J, Ager J, Olson J. Ultrasound differentiation of the competent from the incompetent cervix: prevention of preterm delivery. Am J Obstet Gynecol. 1986;154:537–546. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

