From crystal packing to molecular recognition: prediction and discovery of a binding site on the surface of polo-like kinase 1
- PMID: 21472932
- PMCID: PMC3555362
- DOI: 10.1002/anie.201008019
From crystal packing to molecular recognition: prediction and discovery of a binding site on the surface of polo-like kinase 1
Figures
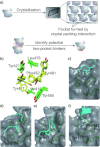
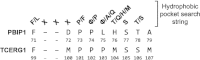
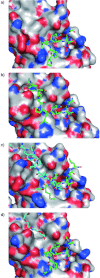
References
-
- Wells JA, McClendon CL. Nature. 2007;450:1001–1009. - PubMed
-
- Whitty A, Kumaravel G. Nat. Chem. Biol. 2006;2:112–118. - PubMed
-
- DeLano WL, Ultsch MH, de Vos AM, Wells JA. Science. 2000;287:1279–1283. - PubMed
-
- Atwell S, Ultsch M, de Vos AM, Wells JA. Science. 1997;278:1125–1128. - PubMed
-
- Keskin O, Gursoy A, Ma B, Nussinov R. Chem. Rev. 2008;108:1225–1244. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

