Design and synthesis of a new class of membrane-permeable triazaborolopyridinium fluorescent probes
- PMID: 21473622
- PMCID: PMC3244355
- DOI: 10.1021/ja2005175
Design and synthesis of a new class of membrane-permeable triazaborolopyridinium fluorescent probes
Abstract
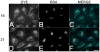
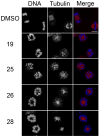
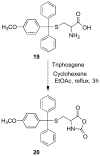
A new class of fluorescent triazaborolopyridinium compounds was synthesized from hydrazones of 2-hydrazinylpyridine (HPY) and evaluated as potential dyes for live-cell imaging applications. The HPY dyes are small, their absorption/emission properties are tunable through variation of pyridyl or hydrazone substituents, and they offer favorable photophysical characteristics featuring large Stokes shifts and general insensitivity to solvent or pH. The stability, neutral charge, cell membrane permeability, and favorable relative influences on the water solubility of HPY conjugates are complementary to existing fluorescent dyes and offer advantages for the development of receptor-targeted small-molecule probes. This potential was assessed through the development of a new class of cysteine-derived HPY-conjugate imaging agents for the kinesin spindle protein (KSP) that is expressed in the cytoplasm during mitosis and is a promising chemotherapeutic target. Conjugates possessing the neutral HPY or charged Alexa Fluor dyes that function as potent, selective allosteric inhibitors of the KSP motor were compared using biochemical and cell-based phenotypic assays and live-cell imaging. These results demonstrate the effectiveness of the HPY dye moiety as a component of probes for an intracellular protein target and highlight the importance of dye structure in determining the pathway of cell entry and the overall performance of small-molecule conjugates as imaging agents.
© 2011 American Chemical Society
Figures





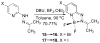

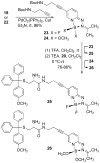

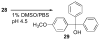
References
-
- Zhang J, Campbell RE, Ting AY, Tsien RY. Nat Rev Mol Cell Bio. 2002;3:906–918. - PubMed
- Lavis LD, Raines RT. ACS Chem Biol. 2008;3(3):142–155. - PMC - PubMed
- Walter NG, Huang CY, Manzo AJ, Sobhy MA. Nature Methods. 2008;5(6):475–489. - PMC - PubMed
- Tinnefeld P, Sauer M. Angew Chem Int Ed. 2005;44:2642–2671. - PubMed
- Goncalves MST. Chem Rev. 2009;109:190–212. - PubMed
- Kobayashi H, Ogawa M, Alford R, Choyke PL, Urano Y. Chem Rev. 2010;110:2620–2640. - PMC - PubMed
-
- Loudet A, Burgess K. Chem Rev. 2007;107(11):4891–4932. - PubMed
-
- Kim E, Koh M, Ryu J, Park SB. J Am Chem Soc. 2008;130:12206–12207. - PubMed
- Abet V, Nuñez A, Mendicuti F, Burgos C, Alvarez-Builla J. J Org Chem. 2008;73:8800–8807. - PubMed
- Ozhalici-Unal H, Pow CL, Marks SA, Jesper LD, Silva GL, Shank NI, Jones EW, Burnette JM, Berget PB, Armitage BA. J Am Chem Soc. 2008;130(38):12620–12621. - PMC - PubMed
-
- Alexander MD, et al. ChemBioChem. 2006;7:409–416. - PubMed
-
- Ramesh C, Bryant B, Nayak T, Revankar CM, Anderson T, Carlson KE, Katzenellenbogen JA, Sklar LA, Norenberg JP, Prossnitz ER, Arterburn JB. J Am Chem Soc. 2006;128:14476–14477. - PMC - PubMed
- Nayak TK, Hathaway HJ, Ramesh C, Arterburn JB, Dai D, Sklar LA, Norenberg JP, Prossnitz ER. J Nuclear Med. 2008;49(6):978–986. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

