Superoxide dismutases: role in redox signaling, vascular function, and diseases
- PMID: 21473702
- PMCID: PMC3151424
- DOI: 10.1089/ars.2011.3999
Superoxide dismutases: role in redox signaling, vascular function, and diseases
Abstract
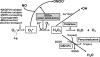
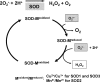
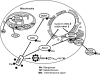
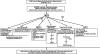
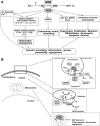
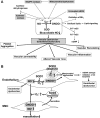
Excessive reactive oxygen species Revised abstract, especially superoxide anion (O₂•-), play important roles in the pathogenesis of many cardiovascular diseases, including hypertension and atherosclerosis. Superoxide dismutases (SODs) are the major antioxidant defense systems against (O₂•-), which consist of three isoforms of SOD in mammals: the cytoplasmic Cu/ZnSOD (SOD1), the mitochondrial MnSOD (SOD2), and the extracellular Cu/ZnSOD (SOD3), all of which require catalytic metal (Cu or Mn) for their activation. Recent evidence suggests that in each subcellular location, SODs catalyze the conversion of (O₂•-), H2O2, which may participate in cell signaling. In addition, SODs play a critical role in inhibiting oxidative inactivation of nitric oxide, thereby preventing peroxynitrite formation and endothelial and mitochondrial dysfunction. The importance of each SOD isoform is further illustrated by studies from the use of genetically altered mice and viral-mediated gene transfer. Given the essential role of SODs in cardiovascular disease, the concept of antioxidant therapies, that is, reinforcement of endogenous antioxidant defenses to more effectively protect against oxidative stress, is of substantial interest. However, the clinical evidence remains controversial. In this review, we will update the role of each SOD in vascular biologies, physiologies, and pathophysiologies such as atherosclerosis, hypertension, and angiogenesis. Because of the importance of metal cofactors in the activity of SODs, we will also discuss how each SOD obtains catalytic metal in the active sites. Finally, we will discuss the development of future SOD-dependent therapeutic strategies.
Figures


References
-
- Abid MR. Tsai JC. Spokes KC. Deshpande SS. Irani K. Aird WC. Vascular endothelial growth factor induces manganese-superoxide dismutase expression in endothelial cells by a Rac1-regulated NADPH oxidase-dependent mechanism. FASEB J. 2001;15:2548–2550. - PubMed
-
- Abreu IA. Cabelli DE. Superoxide dismutases-a review of the metal-associated mechanistic variations. Biochim Biophys Acta. 2010;1804:263–274. - PubMed
-
- Adachi T. Yamazaki N. Tasaki H. Toyokawa T. Yamashita K. Hirano K. Changes in the heparin affinity of extracellular-superoxide dismutase in patients with coronary artery atherosclerosis. Biol Pharm Bull. 1998;21:1090–1093. - PubMed
-
- Agrawal RS. Muangman S. Layne MD. Melo L. Perrella MA. Lee RT. Zhang L. Lopez-Ilasaca M. Dzau VJ. Pre-emptive gene therapy using recombinant adeno-associated virus delivery of extracellular superoxide dismutase protects heart against ischemic reperfusion injury, improves ventricular function and prolongs survival. Gene Ther. 2004;11:962–969. - PubMed
-
- Alvarez B. Demicheli V. Duran R. Trujillo M. Cervenansky C. Freeman BA. Radi R. Inactivation of human Cu,Zn superoxide dismutase by peroxynitrite and formation of histidinyl radical. Free Radic Biol Med. 2004;37:813–822. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

