The zebrafish heart regenerates after cryoinjury-induced myocardial infarction
- PMID: 21473762
- PMCID: PMC3078894
- DOI: 10.1186/1471-213X-11-21
The zebrafish heart regenerates after cryoinjury-induced myocardial infarction
Abstract
Background: In humans, myocardial infarction is characterized by irreversible loss of heart tissue, which becomes replaced with a fibrous scar. By contrast, teleost fish and urodele amphibians are capable of heart regeneration after a partial amputation. However, due to the lack of a suitable infarct model, it is not known how these animals respond to myocardial infarction.
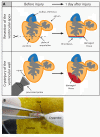
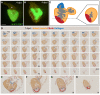
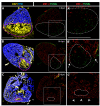
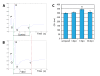
Results: Here, we have established a heart infarct model in zebrafish using cryoinjury. In contrast to the common method of partial resection, cryoinjury results in massive cell death within 20% of the ventricular wall, similar to that observed in mammalian infarcts. As in mammals, the initial stages of the injury response include thrombosis, accumulation of fibroblasts and collagen deposition. However, at later stages, cardiac cells can enter the cell cycle and invade the infarct area in zebrafish. In the subsequent two months, fibrotic scar tissue is progressively eliminated by cell apoptosis and becomes replaced with a new myocardium, resulting in scarless regeneration. We show that tissue remodeling at the myocardial-infarct border zone is associated with accumulation of Vimentin-positive fibroblasts and with expression of an extracellular matrix protein Tenascin-C. Electrocardiogram analysis demonstrated that the reconstitution of the cardiac muscle leads to the restoration of the heart function.
Conclusions: We developed a new cryoinjury model to induce myocardial infarction in zebrafish. Although the initial stages following cryoinjury resemble typical healing in mammals, the zebrafish heart is capable of structural and functional regeneration. Understanding the key healing processes after myocardial infarction in zebrafish may result in identification of the barriers to efficient cardiac regeneration in mammals.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

