The Notch ligand Delta-like 1 integrates inputs from TGFbeta/Activin and Wnt pathways
- PMID: 21473864
- PMCID: PMC3097118
- DOI: 10.1016/j.yexcr.2011.03.019
The Notch ligand Delta-like 1 integrates inputs from TGFbeta/Activin and Wnt pathways
Abstract
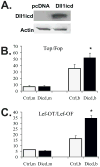
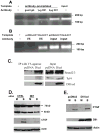
Unlike the well-characterized nuclear function of the Notch intracellular domain, it has been difficult to identify a nuclear role for the ligands of Notch. Here we provide evidence for the nuclear function of the Notch ligand Delta-like 1 in colon cancer (CC) cells exposed to butyrate. We demonstrate that the intracellular domain of Delta-like 1 (Dll1icd) augments the activity of Wnt signaling-dependent reporters and that of the promoter of the connective tissue growth factor (CTGF) gene. Data suggest that Dll1icd upregulates CTGF promoter activity through both direct and indirect mechanisms. The direct mechanism is supported by co-immunoprecipitation of endogenous Smad2/3 proteins and Dll1 and by chromatin immunoprecipitation analyses that revealed the occupancy of Dll1icd on CTGF promoter sequences containing a Smad binding element. The indirect upregulation of CTGF expression by Dll1 is likely due to the ability of Dll1icd to increase Wnt signaling, a pathway that targets CTGF. CTGF expression is induced in butyrate-treated CC cells and results from clonal growth assays support a role for CTGF in the cell growth-suppressive role of butyrate. In conclusion, integration of the Notch, Wnt, and TGFbeta/Activin signaling pathways is in part mediated by the interactions of Dll1 with Smad2/3 and Tcf4.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures





References
-
- Sancho E, Batlle E, Clevers H. Signaling pathways in intestinal development and cancer. Annu Rev Cell Dev Biol. 2004;20:695–723. - PubMed
-
- Morin J, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–1790. - PubMed
-
- Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell Biochem Biophys. 1996;87:159–170. - PubMed
-
- Miyaki M, Iijima T, Kimura J, Yasuno M, Mori T, Hayashi Y, Koike M, Shitara N, Iwama T, Kuroki T. Frequent mutation of beta-catenin and APC genes in primary colorectal tumors from patients with hereditary nonpolyposis colorectal cancer. Cancer Res. 1999;59:4506–4509. - PubMed
-
- Roose J, Clevers H. Tcf transcription factors: molecular switches in carcinogenesis. Biochim Biophys Acta. 1999;87456:M23–M27. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

