Insights into the pathogenesis and treatment of cancer from inborn errors of metabolism
- PMID: 21473982
- PMCID: PMC3071916
- DOI: 10.1016/j.ajhg.2011.03.005
Insights into the pathogenesis and treatment of cancer from inborn errors of metabolism
Abstract
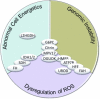
Mutations in genes that play fundamental roles in metabolic pathways have been found to also play a role in tumor development and susceptibility to cancer. At the same time, significant progress has been made in the treatment of patients with inborn errors of metabolism (IEM),(1) resulting in increased longevity and the unmasking of cancer predisposition, frequently hepatocellular carcinoma, in these conditions. These patients offer a potential opportunity to deepen our understanding of how intermediary metabolism impacts tumorigenesis. We provide an overview from the perspective of cancers in patients affected with IEM and discuss how dysregulation of these specific metabolic pathways might contribute to the mechanisms of cancer development and treatment.
Copyright © 2011 The American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Alexander F.E., Patheal S.L., Biondi A., Brandalise S., Cabrera M.E., Chan L.C., Chen Z., Cimino G., Cordoba J.C., Gu L.J. Transplacental chemical exposure and risk of infant leukemia with MLL gene fusion. Cancer Res. 2001;61:2542–2546. - PubMed
-
- Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. - PubMed
-
- Luo J., Manning B.D., Cantley L.C. Targeting the PI3K-Akt pathway in human cancer: Rationale and promise. Cancer Cell. 2003;4:257–262. - PubMed
-
- Lanpher B., Brunetti-Pierri N., Lee B. Inborn errors of metabolism: The flux from Mendelian to complex diseases. Nat. Rev. Genet. 2006;7:449–460. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

