Bone marrow-derived cell therapy stimulates endogenous cardiomyocyte progenitors and promotes cardiac repair
- PMID: 21474103
- PMCID: PMC4148018
- DOI: 10.1016/j.stem.2011.02.002
Bone marrow-derived cell therapy stimulates endogenous cardiomyocyte progenitors and promotes cardiac repair
Erratum in
- Cell Stem Cell. 2015 Jul 2;17(1):125
Abstract
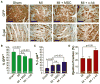
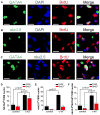
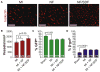
Cell therapy can improve cardiac function in animals and humans after injury, but the mechanism is unclear. We performed cell therapy experiments in genetically engineered mice that permanently express green fluorescent protein (GFP) only in cardiomyocytes after a pulse of 4-OH-tamoxifen. Myocardial infarction diluted the GFP(+) cardiomyocyte pool, indicating refreshment by non-GFP(+) progenitors. Cell therapy with bone marrow-derived c-kit(+) cells, but not mesenchymal stem cells, further diluted the GFP(+) pool, consistent with c-kit(+) cell-mediated augmentation of cardiomyocyte progenitor activity. This effect could not be explained by transdifferentiation to cardiomyocytes by exogenously delivered c-kit(+) cells or by cell fusion. Therapy with c-kit(+) cells but not mesenchymal stem cells improved cardiac function. These findings suggest that stimulation of endogenous cardiogenic progenitor activity is a critical mechanism of cardiac cell therapy.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures


Comment in
-
A repair "kit" for the infarcted heart.Cell Stem Cell. 2011 Apr 8;8(4):350-2. doi: 10.1016/j.stem.2011.03.005. Cell Stem Cell. 2011. PMID: 21474095 Free PMC article.
References
-
- Abdel-Latif A, Bolli R, Tleyjeh IM, Montori VM, Perin EC, Hornung CA, Zuba-Surma EK, Al-Mallah M, Dawn B. Adult bone marrow-derived cells for cardiac repair: A systematic review and meta-analysis. Arch Intern Med. 2007;167:989–997. - PubMed
-
- Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428:668–673. - PubMed
-
- Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. - PubMed
-
- Beltrami AP, Cesselli D, Bergamin N, Marcon P, Rigo S, Puppato E, D’Aurizio F, Verardo R, Piazza S, Pignatelli A, et al. Multipotent cells can be generated in vitro from several adult human organs (heart, liver, and bone marrow) Blood. 2007;110:3438–3446. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

