Ion transport mechanisms linked to bicarbonate secretion in the esophageal submucosal glands
- PMID: 21474426
- PMCID: PMC3129871
- DOI: 10.1152/ajpregu.00648.2010
Ion transport mechanisms linked to bicarbonate secretion in the esophageal submucosal glands
Abstract
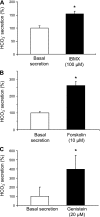
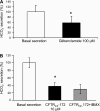
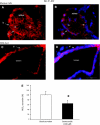
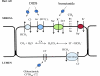
The esophageal submucosal glands (SMG) secrete HCO(3)(-) and mucus into the esophageal lumen, where they contribute to acid clearance and epithelial protection. This study characterized the ion transport mechanisms linked to HCO(3)(-) secretion in SMG. We localized ion transporters using immunofluorescence, and we examined their expression by RT-PCR and in situ hybridization. We measured HCO(3)(-) secretion by using pH stat and the isolated perfused esophagus. Using double labeling with Na(+)-K(+)-ATPase as a marker, we localized Na(+)-coupled bicarbonate transporter (NBCe1) and Cl(-)-HCO(3)(-) exchanger (SLC4A2/AE2) to the basolateral membrane of duct cells. Expression of cystic fibrosis transmembrane regulator channel (CFTR) was confirmed by immunofluorescence, RT-PCR, and in situ hybridization. We identified anion exchanger SLC26A6 at the ducts' luminal membrane and Na(+)-K(+)-2Cl(-) (NKCC1) at the basolateral membrane of mucous and duct cells. pH stat experiments showed that elevations in cAMP induced by forskolin or IBMX increased HCO(3)(-) secretion. Genistein, an activator of CFTR, which does not increase intracellular cAMP, also stimulated HCO(3)(-) secretion, whereas glibenclamide, a Cl(-) channel blocker, and bumetanide, a Na(+)-K(+)-2Cl(-) blocker, decreased it. CFTR(inh)-172, a specific CFTR channel blocker, inhibited basal HCO(3)(-) secretion as well as stimulation of HCO(3)(-) secretion by IBMX. This is the first report on the presence of CFTR channels in the esophagus. The role of CFTR in manifestations of esophageal disease in cystic fibrosis patients remains to be determined.
Figures






References
-
- Abdulnour-Nakhoul S, Nakhoul NL, Orlando RC. Lumen-to-surface pH gradients in opossum and rabbit esophagi: role of submucosal glands. Am J Physiol Gastrointest Liver Physiol 278: G113–G120, 2000 - PubMed
-
- Abdulnour-Nakhoul S, Nakhoul NL, Wheeler SA, Wang P, Swenson ER, Orlando RC. HCO3− secretion in the esophageal submucosal glands. Am J Physiol Gastrointest Liver Physiol 288: G736–G744, 2005 - PubMed
-
- Abdulnour-Nakhoul S, Nakhoul NL, Wheeler SA, Haque S, Wang P, Brown K, Orlando GS, Orlando RC. Characterization of esophageal submucosal glands in pig tissue and cultures. Dig Dis Sci 52: 3054–3065, 2007 - PubMed
-
- Abuladze N, Lee I, Newman D, Hwang J, Boorer K, Pushkin A, Kurtz I. Molecular cloning, chromosomal localization, tissue distribution, and functional expression of the human pancreatic sodium bicarbonate cotransporter. J Biol Chem 273: 17689–17695, 1998 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

