Positive feedback regulation between gamma-aminobutyric acid type A (GABA(A)) receptor signaling and brain-derived neurotrophic factor (BDNF) release in developing neurons
- PMID: 21474450
- PMCID: PMC3122223
- DOI: 10.1074/jbc.M110.201582
Positive feedback regulation between gamma-aminobutyric acid type A (GABA(A)) receptor signaling and brain-derived neurotrophic factor (BDNF) release in developing neurons
Abstract
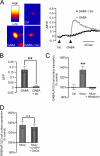
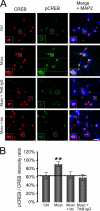
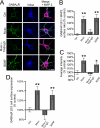
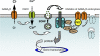
During the early development of the nervous system, γ-aminobutyric acid (GABA) type A receptor (GABA(A)R)-mediated signaling parallels the neurotrophin/tropomyosin-related kinase (Trk)-dependent signaling in controlling a number of processes from cell proliferation and migration, via dendritic and axonal outgrowth, to synapse formation and plasticity. Here we present the first evidence that these two signaling systems regulate each other through a complex positive feedback mechanism. We first demonstrate that GABA(A)R activation leads to an increase in the cell surface expression of these receptors in cultured embryonic cerebrocortical neurons, specifically at the stage when this activity causes depolarization of the plasma membrane and Ca(2+) influx through L-type voltage-gated Ca(2+) channels. We further demonstrate that GABA(A)R activity triggers release of the brain-derived neurotrophic factor (BDNF), which, in turn by activating TrkB receptors, mediates the observed increase in cell surface expression of GABA(A)Rs. This BDNF/TrkB-dependent increase in surface levels of GABA(A)Rs requires the activity of phosphoinositide 3-kinase (PI3K) and protein kinase C (PKC) and does not involve the extracellular signal-regulated kinase (ERK) 1/2 activity. The increase in GABA(A)R surface levels occurs due to an inhibition of the receptor endocytosis by BDNF, whereas the receptor reinsertion into the plasma membrane remains unaltered. Thus, GABA(A)R activity is a potent regulator of the BDNF release during neuronal development, and at the same time, it is strongly enhanced by the activity of the BDNF/TrkB/PI3K/PKC signaling pathway.
Figures





References
-
- Farrant M., Kaila K. (2007) Prog. Brain Res. 160, 59–87 - PubMed
-
- Whiting P. J., Bonnert T. P., McKernan R. M., Farrar S., Le Bourdellès B., Heavens R. P., Smith D. W., Hewson L., Rigby M. R., Sirinathsinghji D. J., Thompson S. A., Wafford K. A. (1999) Ann. N.Y. Acad. Sci. 868, 645–653 - PubMed
-
- LoTurco J. J., Owens D. F., Heath M. J., Davis M. B., Kriegstein A. R. (1995) Neuron 15, 1287–1298 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

