Cyclooxygenase-2 regulates Th17 cell differentiation during allergic lung inflammation
- PMID: 21474648
- PMCID: PMC3172888
- DOI: 10.1164/rccm.201010-1637OC
Cyclooxygenase-2 regulates Th17 cell differentiation during allergic lung inflammation
Abstract
Rationale: Th17 cells comprise a distinct lineage of proinflammatory T helper cells that are major contributors to allergic responses. It is unknown whether cyclooxygenase (COX)-derived eicosanoids regulate Th17 cells during allergic lung inflammation.
Objectives: To determine the role of COX metabolites in regulating Th17 cell differentiation and function during allergic lung inflammation.
Methods: COX-1(-/-), COX-2(-/-), and wild-type mice were studied in an in vivo model of ovalbumin-induced allergic inflammation and an in vitro model of Th17 differentiation using flow cytometry, cytokine assays, confocal microscopy, real-time polymerase chain reaction, and immunoblotting. In addition, the role of specific eicosanoids and their receptors was examined using synthetic prostaglandins (PGs), selective inhibitors, and siRNA knockdown.
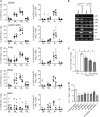
Measurements and main results: Th17 cell differentiation in lung, lymph nodes, and bronchoalveolar lavage fluid was significantly lower in COX-2(-/-) mice after ovalbumin sensitization and exposure in vivo. In vitro studies revealed significantly impaired Th17 cell differentiation of COX-2(-/-) naive CD4(+) T cells with decreased Stat3 phosphorylation and RORγt expression. Synthetic PGF(2α) and PGI(2) enhanced Th17 cell differentiation of COX-2(-/-) CD4(+) T cells in vitro. The selective COX-2 inhibitor, NS-398, and PGF(2α) receptor and PGI(2) receptor siRNA knockdown significantly decreased Th17 cell differentiation in vitro. Administration of synthetic PGs restored accumulation of Th17 cells in lungs of allergic COX-2(-/-) mice in vivo.
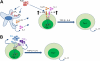
Conclusions: COX-2 is a critical regulator of Th17 cell differentiation during allergic lung inflammation via autocrine signaling of PGI(2) and PGF(2α) through their respective cell surface receptors.
Figures






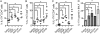
Comment in
-
The ever-expanding role of prostanoids in regulating immune responses.Am J Respir Crit Care Med. 2011 Jul 1;184(1):1-2. doi: 10.1164/rccm.201104-0622ED. Am J Respir Crit Care Med. 2011. PMID: 21737587 No abstract available.
-
PGI2-induced Th17 cell differentiation in connective tissue disease: a comment.Ann Rheum Dis. 2013 Jul;72(7):e15. doi: 10.1136/annrheumdis-2013-203555. Epub 2013 Apr 20. Ann Rheum Dis. 2013. PMID: 23606707 No abstract available.
References
-
- Annunziato F, Cosmi L, Liotta F, Maggi E, Romagnani S. Human Th17 cells: are they different from murine Th17 cells? Eur J Immunol 2009;39:637–640 - PubMed
-
- Iwakura Y, Nakae S, Saijo S, Ishigame H. The roles of IL-17A in inflammatory immune responses and host defense against pathogens. Immunol Rev 2008;226:57–79 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

