Selected Background Findings and Interpretation of Common Lesions in the Female Reproductive System in Macaques
- PMID: 21475639
- PMCID: PMC3070965
- DOI: 10.1177/0192623308327117
Selected Background Findings and Interpretation of Common Lesions in the Female Reproductive System in Macaques
Abstract
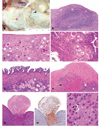
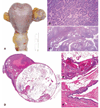
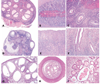
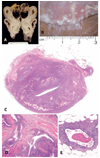
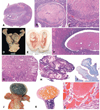
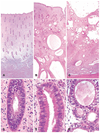
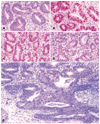
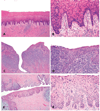
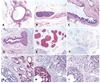
The authors describe a selection of normal findings and common naturally occurring lesions in the reproductive system of female macaques, including changes in the ovaries, uterus, cervix, vagina, and mammary glands. Normal features of immature ovaries, uteri, and mammary glands are described. Common non-neoplastic lesions in the ovaries include cortical mineralization, polyovular follicles, cysts, ovarian surface epithelial hyperplasia, and ectopic ovarian tissue. Ovarian neoplasms include granulosa cell tumors, teratomas, and ovarian surface epithelial tumors. Common non-neoplastic uterine findings include loss of features of normal cyclicity, abnormal bleeding, adenomyosis, endometriosis, epithelial plaques, and pregnancy-associated vascular remodeling. Hyperplastic and neoplastic lesions of the uterus include endometrial polyps, leiomyomas, and rarely endometrial hyperplasia and endometrial adenocarcinoma. Vaginitis is common. Cervical lesions include endocervical squamous metaplasia, polyps, and papillomavirus-associated lesions. Lesions in the mammary gland are most often proliferative and range from ductal hyperplasia to invasive carcinoma. Challenges to interpretation include the normal or pathologic absence of menstrual cyclicity and the potential misinterpretation of sporadic lesions, such as epithelial plaques or papillomavirus-associated lesions. Interpretation of normal and pathologic findings is best accomplished with knowledge of the life stage, reproductive history, and hormonal status of the animal.
Figures




References
-
- Abdel-Fatah TM, Powe DG, Hodi Z, Lee AH, Reis-Filho JS, Ellis IO. High frequency of coexistence of columnar cell lesions, lobular neoplasia, and low grade ductal carcinoma in situ with invasive tubular carcinoma and invasive lobular carcinoma. Am J Surg Pathol. 2007;31:417–426. - PubMed
-
- Adams MR, Kaplan JR, Koritnik DR. Psychosocial influences on ovarian endocrine and ovulatory function in Macaca fascicularis. Physiol Behav. 1985;35:935–940. - PubMed
-
- Ami Y, Suzaki Y, Goto N. Endometriosis in cynomolgus monkeys retired from breeding. J Vet Med Sci. 1993;55:7–11. - PubMed
-
- Arifin E, Shively CA, Register TC, Cline JM. Polycystic ovary syndrome with endometrial hyperplasia in a cynomolgus monkey (Macaca fascicularis) Veterinary Pathology. in press. - PubMed
-
- Ashbeck EL, Rosenberg RD, Stauber PM, Key CR. Benign breast biopsy diagnosis and subsequent risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:467–472. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

