Oxidative photoreceptor cell damage in autoimmune uveitis
- PMID: 21475655
- PMCID: PMC3062768
- DOI: 10.1007/s12348-010-0007-5
Oxidative photoreceptor cell damage in autoimmune uveitis
Abstract
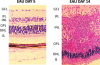
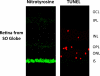
Uveitis comprises an extensive array of intraocular inflammatory diseases and often results in irreversible visual loss. Experimental autoimmune uveitis (EAU) is an animal model used to study human uveitis. Both innate and adaptive immune responses are known to mediate retinal damage in EAU. The innate immune response occurs first with activation of toll-like receptors which upregulate inflammatory cytokines, leading to oxidative stress; subsequently, the adaptive immune response results in inflammatory cytokine upregulation and mitochondrial oxidative stress. In early EAU, mitochondrial DNA is damaged before inflammatory cellular infiltration and alters mitochondrial protein levels and the functions of mitochondria in AU. Our recent study confirms the importance of TLR4 in the generation of inflammatory cytokines, initiation of oxidative DNA damage, and induction of mitochondrial oxidative stress. Like EAU, sympathetic ophthalmia also results in photoreceptor mitochondrial oxidative damage. Agents that prevent mitochondrial oxidative stress and photoreceptor apoptosis may help prevent retinal damage and preserve vision in uveitis.
Keywords: Complete Freund’s adjuvant; Experimental autoimmune uveitis; Mitochondrial oxidative stress; Sympathetic ophthalmia; TLR4.
Figures

Similar articles
-
Mitochondrial oxidative stress initiates visual loss in sympathetic ophthalmia.Jpn J Ophthalmol. 2012 May;56(3):191-7. doi: 10.1007/s10384-012-0132-9. Epub 2012 Apr 3. Jpn J Ophthalmol. 2012. PMID: 22476625 Review.
-
The role of TLR4 in photoreceptor {alpha}a crystallin upregulation during early experimental autoimmune uveitis.Invest Ophthalmol Vis Sci. 2010 Jul;51(7):3680-6. doi: 10.1167/iovs.09-4575. Epub 2010 Mar 5. Invest Ophthalmol Vis Sci. 2010. PMID: 20207969 Free PMC article.
-
Mitochondrial oxidative DNA damage in experimental autoimmune uveitis.Invest Ophthalmol Vis Sci. 2008 Aug;49(8):3299-304. doi: 10.1167/iovs.07-1607. Epub 2008 Apr 30. Invest Ophthalmol Vis Sci. 2008. PMID: 18450595
-
microRNA 146a ameliorates retinal damage in experimental autoimmune uveitis.Front Ophthalmol (Lausanne). 2023 Mar 24;3:1130202. doi: 10.3389/fopht.2023.1130202. eCollection 2023. Front Ophthalmol (Lausanne). 2023. PMID: 38983073 Free PMC article.
-
Photoreceptor mitochondrial oxidative stress in experimental autoimmune uveitis.Ophthalmic Res. 2008;40(3-4):160-4. doi: 10.1159/000119869. Epub 2008 Apr 18. Ophthalmic Res. 2008. PMID: 18421232 Review.
Cited by
-
Hyaluronic acid-curcumin nanoparticles for preventing the progression of experimental autoimmune uveitis through the Keap1/Nrf2/HO-1 signaling pathway.J Nanobiotechnology. 2025 Feb 7;23(1):89. doi: 10.1186/s12951-024-03082-3. J Nanobiotechnology. 2025. PMID: 39915858 Free PMC article.
-
Aryl Hydrocarbon Receptor Regulates Apoptosis and Inflammation in a Murine Model of Experimental Autoimmune Uveitis.Front Immunol. 2018 Jul 25;9:1713. doi: 10.3389/fimmu.2018.01713. eCollection 2018. Front Immunol. 2018. PMID: 30090104 Free PMC article.
-
Role of the retinal vascular endothelial cell in ocular disease.Prog Retin Eye Res. 2013 Jan;32:102-80. doi: 10.1016/j.preteyeres.2012.08.004. Epub 2012 Sep 11. Prog Retin Eye Res. 2013. PMID: 22982179 Free PMC article. Review.
-
Crystallin β-b2 promotes retinal ganglion cell protection in experimental autoimmune uveoretinitis.Front Cell Neurosci. 2024 Sep 10;18:1379540. doi: 10.3389/fncel.2024.1379540. eCollection 2024. Front Cell Neurosci. 2024. PMID: 39318470 Free PMC article.
-
Role of microglia/macrophage polarisation in intraocular diseases (Review).Int J Mol Med. 2024 May;53(5):45. doi: 10.3892/ijmm.2024.5369. Epub 2024 Mar 29. Int J Mol Med. 2024. PMID: 38551157 Free PMC article. Review.
References
-
- Caspi RR (2003) Experimental autoimmune uveoretinitis in the rat and mouse. Curr Protoc Immunol Chapter 15:Unit 15.6. doi:10.1002/0471142735.im1506s53 - PubMed
LinkOut - more resources
Full Text Sources

