A convergent route to the CDEF-tetracycle of pectenotoxin-2
- PMID: 21476517
- PMCID: PMC3156112
- DOI: 10.1021/ol200627d
A convergent route to the CDEF-tetracycle of pectenotoxin-2
Abstract
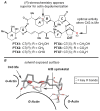
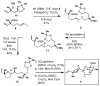
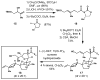
A convergent synthesis of the CDEF-tetracyclic region of pectenotoxin-2 (PTX-2) is described. The synthetic pathway derives from a head-to-tail union of 2 equiv of linalool to establish a stereodefined DEF-tricyclic aldehyde. Subsequent coupling with a "northern" fragment enolate, followed by a tandem Sharpless epoxidation/cyclization, delivers the C10-C26 polycyclic region of the natural product.
Figures



References
-
- Yasumoto T, Murate M, Oshima Y, Sano M, Matsumoto GK, Clardy J. Tetrahedron. 1985;41:1019–1025.
- Sasaki K, Wright JLC, Yasumoto T. J Org Chem. 1998;63:2475–2480. - PubMed
-
-
Espina B, Louzao MC, Ares IR, Fonfría EW, Vilarino N, Vieytes MR, Yasumoto T, Botana LM. Chem Res Toxicol. 2010;23:504–515.Ares I, Louzao C, Espina B, Vieytes MR, Miles CO, Yasumoto T, Botana L. Cell Physiol Biochem. 2007;19:283–292.(c) see also ref. 4a.
-
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

