Cardiolipin deficiency in Rhodobacter sphaeroides alters the lipid profile of membranes and of crystallized cytochrome oxidase, but structure and function are maintained
- PMID: 21476578
- PMCID: PMC3097902
- DOI: 10.1021/bi101702c
Cardiolipin deficiency in Rhodobacter sphaeroides alters the lipid profile of membranes and of crystallized cytochrome oxidase, but structure and function are maintained
Abstract
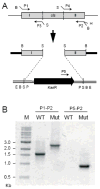
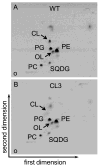
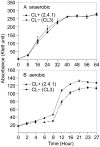
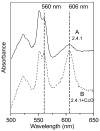
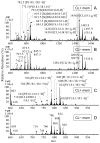
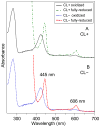
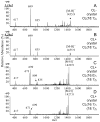
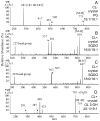
Many recent studies highlight the importance of lipids in membrane proteins, including in the formation of well-ordered crystals. To examine the effect of changes in one lipid, cardiolipin, on the lipid profile and the production, function, and crystallization of an intrinsic membrane protein, cytochrome c oxidase, we mutated the cardiolipin synthase (cls) gene of Rhodobacter sphaeroides, causing a >90% reduction in cardiolipin content in vivo and selective changes in the abundances of other lipids. Under these conditions, a fully native cytochrome c oxidase (CcO) was produced, as indicated by its activity, spectral properties, and crystal characteristics. Analysis by MALDI tandem mass spectrometry (MS/MS) revealed that the cardiolipin level in CcO crystals, as in the membranes, was greatly decreased. Lipid species present in the crystals were directly analyzed for the first time using MS/MS, documenting their identities and fatty acid chain composition. The fatty acid content of cardiolipin in R. sphaeroides CcO (predominantly 18:1) differs from that in mammalian CcO (18:2). In contrast to the cardiolipin dependence of mammalian CcO activity, major depletion of cardiolipin in R. sphaeroides did not impact any aspect of CcO structure or behavior, suggesting a greater tolerance of interchange of cardiolipin with other lipids in this bacterial system.
Figures

References
-
- Nussberger S, Doerr K, Wang DN, Kuhlbrandt W. Lipid-protein interactions in crystals of plant light-harvesting complex. J Mol Biol. 1993;234:347–356. - PubMed
-
- Bogdanov M, Sun J, Kaback HR, Dowhan W. A phospholipid acts as a chaperone in assembly of a membrane transport protein. J Biol Chem. 1996;271:11615–11618. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

