Impact of pre-existing immunity on gene transfer to nonhuman primate liver with adeno-associated virus 8 vectors
- PMID: 21476868
- PMCID: PMC3225046
- DOI: 10.1089/hum.2011.031
Impact of pre-existing immunity on gene transfer to nonhuman primate liver with adeno-associated virus 8 vectors
Abstract
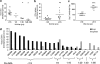
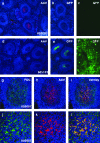
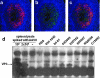
Vectors based on the primate-derived adeno-associated virus serotype 8 (AAV8) are being evaluated in preclinical and clinical models. Natural infections with related AAVs activate memory B cells that produce antibodies capable of modulating the efficacy and safety of the vector. We have evaluated the biology of AAV8 gene transfer in macaque liver, with a focus on assessing the impact of pre-existing humoral immunity. Twenty-one macaques with various levels of AAV neutralizing antibody (NAb) were injected intravenously with AAV8 vector expressing green fluorescent protein. Pre-existing antibody titers in excess of 1:10 substantially diminished hepatocyte transduction that, in the absence of NAbs, was highly efficient. Vector-specific NAb diminished liver deposition of genomes and unexpectedly increased genome distribution to the spleen. The majority of animals showed high-level and stable sequestration of vector capsid protein by follicular dendritic cells of splenic germinal centers. These studies illustrate how natural immunity to a virus that is related to a vector can impact the efficacy and potential safety of in vivo gene therapy. We propose to use the in vitro transduction inhibition assay to evaluate research subjects before gene therapy and to preclude from systemic AAV8 trials those that have titers in excess of 1:10.
Figures




References
-
- Bainbridge J.W. Smith A.J. Barker S.S., et al. Effect of gene therapy on visual function in Leber's congenital amaurosis. N. Engl. J. Med. 2008;358:2231–2239. - PubMed
-
- Bell P. Moscioni A.D. McCarter R.J., et al. Analysis of tumors arising in male B6C3F1 mice with and without AAV vector delivery to liver. Mol. Ther. 2006;14:34–44. - PubMed
-
- Boutin S. Monteilhet V. Veron P., et al. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: Implications for gene therapy using AAV vectors. Hum. Gene Ther. 2010;21:704–712. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

