The availability and age-appropriateness of medicines authorized for children in The Netherlands
- PMID: 21477143
- PMCID: PMC3175516
- DOI: 10.1111/j.1365-2125.2011.03982.x
The availability and age-appropriateness of medicines authorized for children in The Netherlands
Abstract
Aim: To study the number of medicines and active chemical entities that are authorized and commercially available for children in the Netherlands and to evaluate the age-appropriateness of the available paediatric medicines.
Methods: The availability of paediatric medicines and active chemical entities was studied with the help of a Dutch medicines database and the Summary of Product Characteristics. Medicines were categorized with respect to their route of administration, type of oral dosage form and therapeutic category. The age-appropriateness was assessed on three aspects: dose capability, suitability of the dosage form and inclusion of potentially harmful excipients.
Results: Three thousand five hundred and forty-two paediatric medicines containing 703 different active chemical entities were identified. This equalled half of all the medicines and chemical entities available for human use. The percentage of paediatric medicines increased with age and varied for the route of administration from 22% (dermal) to 81% (inhalation) and for the therapeutic category from 11% (uro-genital, sex hormones) to 89% (anti-parasites). The appropriateness of the paediatric medicines with respect to their authorization status, dose capability and dosage form increased with age from 27-88%. Fifty-two percent of all oral paediatric liquid formulations contained a potentially harmful excipient.
Conclusion: This study confirms the limited availability of paediatric medicines for a broad range of therapeutic areas and shows that paediatric medicines may not be age-appropriate, even if authorized. While confirming the need for a legislative incentive, the results also provide baseline information for an estimation of the effect of the European Paediatric Regulation in the near future.
© 2011 The Authors. British Journal of Clinical Pharmacology © 2011 The British Pharmacological Society.
Figures
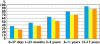
 ); authorized and able to provide for the recommended dose (dose capable) (
); authorized and able to provide for the recommended dose (dose capable) ( ); authorized, dose capable and having a suitable dosage form (
); authorized, dose capable and having a suitable dosage form ( )
)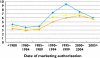
 ); authorized and dose capable (
); authorized and dose capable ( ); authorized, dose capable and having a suitable dosage form (
); authorized, dose capable and having a suitable dosage form ( )
)References
-
- Balakrishnan K, Grieve J, Tordoff J, Norris P, Reith D. Pediatric licensing status and the availability of suitable formulations for new medical entities approved in the United States between 1998 and 2002. J Clin Pharmacol. 2006;46:1038–43. - PubMed
-
- Ceci A, Felisi M, Baiardi P, Bonifazi F, Catapano M, Giaquinto C, Nicolosi A, Sturkenboom M, Neubert A, Wong I. Medicines for children licensed by the European Medicines Agency (EMEA): the balance after 10 years. Eur J Clin Pharmacol. 2006;62:947–52. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

