Quantitative proteomics of yeast post-Golgi vesicles reveals a discriminating role for Sro7p in protein secretion
- PMID: 21477180
- PMCID: PMC3926324
- DOI: 10.1111/j.1600-0854.2011.01186.x
Quantitative proteomics of yeast post-Golgi vesicles reveals a discriminating role for Sro7p in protein secretion
Abstract
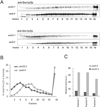
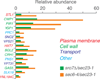
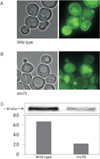
We here report the first comparative proteomics of purified yeast post-Golgi vesicles (PGVs). Vesicle samples isolated from PGV-accumulating sec6-4 mutants were treated with isobaric tags (iTRAQ) for subsequent quantitative tandem mass spectrometric analysis of protein content. After background subtraction, a total of 66 vesicle-associated proteins were identified, including known or assumed vesicle residents as well as a fraction not previously known to be PGV associated. Vesicles isolated from cells lacking the polarity protein Sro7p contained essentially the same catalogue of proteins but showed a reduced content of a subset of cargo proteins, in agreement with a previously shown selective role for Sro7p in cargo sorting.
© 2011 John Wiley & Sons A/S.
Figures



Similar articles
-
The yeast tumor suppressor homologue Sro7p is required for targeting of the sodium pumping ATPase to the cell surface.Mol Biol Cell. 2006 Dec;17(12):4988-5003. doi: 10.1091/mbc.e05-08-0798. Epub 2006 Sep 27. Mol Biol Cell. 2006. PMID: 17005914 Free PMC article.
-
Yeast homologues of lethal giant larvae and type V myosin cooperate in the regulation of Rab-dependent vesicle clustering and polarized exocytosis.Mol Biol Cell. 2011 Mar 15;22(6):842-57. doi: 10.1091/mbc.E10-07-0570. Epub 2011 Jan 19. Mol Biol Cell. 2011. PMID: 21248204 Free PMC article.
-
Yeast homologues of tomosyn and lethal giant larvae function in exocytosis and are associated with the plasma membrane SNARE, Sec9.J Cell Biol. 1999 Jul 12;146(1):125-40. doi: 10.1083/jcb.146.1.125. J Cell Biol. 1999. PMID: 10402465 Free PMC article.
-
Protein transport from the late Golgi to the vacuole in the yeast Saccharomyces cerevisiae.Biochim Biophys Acta. 2005 Jul 10;1744(3):438-54. doi: 10.1016/j.bbamcr.2005.04.004. Biochim Biophys Acta. 2005. PMID: 15913810 Review.
-
An Overview of Protein Secretion in Yeast and Animal Cells.Methods Mol Biol. 2017;1662:1-17. doi: 10.1007/978-1-4939-7262-3_1. Methods Mol Biol. 2017. PMID: 28861813 Review.
Cited by
-
High-resolution secretory timeline from vesicle formation at the Golgi to fusion at the plasma membrane in S. cerevisiae.Elife. 2022 Nov 4;11:e78750. doi: 10.7554/eLife.78750. Elife. 2022. PMID: 36331188 Free PMC article.
-
Quantitative analysis of membrane trafficking in regulation of Cdc42 polarity.Traffic. 2014 Dec;15(12):1330-43. doi: 10.1111/tra.12211. Epub 2014 Oct 8. Traffic. 2014. PMID: 25158298 Free PMC article.
-
Quantitative Analyses of the Yeast Oxidative Protein Folding Pathway In Vitro and In Vivo.Antioxid Redox Signal. 2019 Aug 1;31(4):261-274. doi: 10.1089/ars.2018.7615. Epub 2019 Apr 25. Antioxid Redox Signal. 2019. PMID: 30880408 Free PMC article.
-
Involvement of the exomer complex in the polarized transport of Ena1 required for Saccharomyces cerevisiae survival against toxic cations.Mol Biol Cell. 2017 Dec 1;28(25):3672-3685. doi: 10.1091/mbc.E17-09-0549. Epub 2017 Oct 11. Mol Biol Cell. 2017. PMID: 29021337 Free PMC article.
-
Yeast Rgd3 is a phospho-regulated F-BAR-containing RhoGAP involved in the regulation of Rho3 distribution and cell morphology.Mol Biol Cell. 2020 Nov 1;31(23):2570-2582. doi: 10.1091/mbc.E20-05-0288. Epub 2020 Sep 17. Mol Biol Cell. 2020. PMID: 32941095 Free PMC article.
References
-
- Pruyne D, Legesse-Miller A, Gao L, Dong Y, Bretscher A. Mechanisms of polarized growth and organelle segregation in yeast. Annu Rev Cell Dev Biol. 2004;20:559–591. - PubMed
-
- Hsu SC, TerBush D, Abraham M, Guo W. The exocyst complex in polarized exocytosis. Int Rev Cytol. 2004;233:243–265. - PubMed
-
- Brennwald P, Kearns B, Champion K, Keranen S, Bankaitis V, Novick P. Sec9 is a SNAP-25-like component of a yeast SNARE complex that may be the effector of Sec4 function in exocytosis. Cell. 1994;79:245–258. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

