Community-based cross-sectional survey of latent tuberculosis infection in Afar pastoralists, Ethiopia, using QuantiFERON-TB Gold In-Tube and tuberculin skin test
- PMID: 21477326
- PMCID: PMC3080306
- DOI: 10.1186/1471-2334-11-89
Community-based cross-sectional survey of latent tuberculosis infection in Afar pastoralists, Ethiopia, using QuantiFERON-TB Gold In-Tube and tuberculin skin test
Abstract
Background: There is little information concerning community-based prevalence of latent tuberculosis infection (LTBI) using T-cell based interferon-γ (IFN-γ) release assays (IGRAs), particularly in TB endemic settings. In this study, the prevalence of LTBI in the Afar pastoral community was assessed using QuantiFERON-TB Gold In-Tube (QFTGIT) and tuberculin skin tests (TST).
Methods: A community-based cross-sectional survey of LTBI involving 652 apparently healthy adult pastoralists was undertaken in the pastoral community of Amibara District of the Afar Region between April and June 2010.
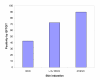
Results: The prevalence of LTBI was estimated as 63.7% (363/570) using QFTGIT at the cut-off point recommended by the manufacturer (≥0.35 IU/ml IFN-γ), while it was 74.9% (427/570) using a cut-off point≥0.1 IU/ml IFN-γ. The QFTGIT-based prevalence of LTBI was not significantly associated with the gender or age of the study participants. However, the prevalence of LTBI was 31.2% (183/587) using TST at a cut-off point≥10 mm of skin indurations, and it was higher in males than females (36.8% vs. 23.5%, X2=11.76; p<0.001). There was poor agreement between the results of the tests (k=0.098, 95% CI, 0.08-0.13). However, there was a positive trend between QFTGIT and TST positivity (X2=96.76, P<0.001). Furthermore, individuals with skin indurations≥10 mm were 13.6 times more likely to have positive results using QFTGIT than individuals with skin indurations of 0 mm (adjusted OR=13.6; 95%CI, 7.5 to 24.7, p<0.001).
Conclusions: There is currently no agreed gold standard for diagnosis of LTBI. However, the higher prevalence of LTBI detected using QFTGIT rather than TST suggests that QFTGIT could be used for epidemiological studies concerning LTBI at the community level, even in a population unreactive to TST. Further studies of adults and children will be required to assess the effects of factors such as malnutrition, non-tuberculosis mycobacterial infections, HIV and parasitic infections on the performance of QFTGIT.
Figures
Similar articles
-
Performance of QuantiFERON-TB Gold In-Tube (QFTGIT) for the diagnosis of Mycobacterium tuberculosis (Mtb) infection in Afar Pastoralists, Ethiopia.BMC Infect Dis. 2010 Dec 17;10:354. doi: 10.1186/1471-2334-10-354. BMC Infect Dis. 2010. PMID: 21162756 Free PMC article.
-
Effect of pregnancy and HIV infection on detection of latent TB infection by Tuberculin Skin Test and QuantiFERON-TB Gold In-Tube assay among women living in a high TB and HIV burden setting.Int J Infect Dis. 2020 Dec;101:235-242. doi: 10.1016/j.ijid.2020.09.1452. Epub 2020 Oct 9. Int J Infect Dis. 2020. PMID: 33039610 Free PMC article.
-
Association of the level of IFN-γ produced by T cells in response to Mycobacterium tuberculosis-specific antigens with the size of skin test indurations among individuals with latent tuberculosis in a highly tuberculosis-endemic setting.Int Immunol. 2012 Feb;24(2):71-8. doi: 10.1093/intimm/dxr102. Epub 2012 Jan 31. Int Immunol. 2012. PMID: 22298884
-
Interferon-γ release assays or tuberculin skin test for detection and management of latent tuberculosis infection: a systematic review and meta-analysis.Lancet Infect Dis. 2020 Dec;20(12):1457-1469. doi: 10.1016/S1473-3099(20)30276-0. Epub 2020 Jul 13. Lancet Infect Dis. 2020. PMID: 32673595
-
Interferon gamma release assays: principles and practice.Enferm Infecc Microbiol Clin. 2010 Apr;28(4):245-52. doi: 10.1016/j.eimc.2009.05.012. Epub 2009 Sep 24. Enferm Infecc Microbiol Clin. 2010. PMID: 19783328 Review.
Cited by
-
Tuberculin skin test positivity among HIV-infected alcohol drinkers on antiretrovirals in south-western Uganda.PLoS One. 2020 Jul 2;15(7):e0235261. doi: 10.1371/journal.pone.0235261. eCollection 2020. PLoS One. 2020. PMID: 32614873 Free PMC article.
-
Diagnosis of latent tuberculosis infection in healthy young adults in a country with high tuberculosis burden and BCG vaccination at birth.BMC Res Notes. 2012 Aug 7;5:415. doi: 10.1186/1756-0500-5-415. BMC Res Notes. 2012. PMID: 22870897 Free PMC article.
-
Prevalence of latent tuberculosis infection and associated risk factors in an urban African setting.BMC Infect Dis. 2015 Mar 29;15:165. doi: 10.1186/s12879-015-0904-1. BMC Infect Dis. 2015. PMID: 25879423 Free PMC article.
-
Latent tuberculosis infection and associated risk factors among people living with HIV and apparently healthy blood donors at the University of Gondar referral hospital, Northwest Ethiopia.BMC Res Notes. 2019 Aug 16;12(1):515. doi: 10.1186/s13104-019-4548-x. BMC Res Notes. 2019. PMID: 31420007 Free PMC article.
-
Helminth infection increases the probability of indeterminate QuantiFERON gold in tube results in pregnant women.Biomed Res Int. 2014;2014:364137. doi: 10.1155/2014/364137. Epub 2014 Feb 19. Biomed Res Int. 2014. PMID: 24701572 Free PMC article.
References
-
- WHO. Global Tuberculosis Control, Epidemiology, Strategy. Geneva, Switzerland: WHO report; 2009.
-
- WHO. The world health report 1999. Geneva: WHO; 1999. p. 110.
-
- Farhat M, Greenaway C, Pai M, Menzies D. False-positive tuberculin skin tests: what is the absolute effect of BCG and non-tuberculous mycobacteria? Int J Tuberc Lung Dis. 2006;10:1192–1204. - PubMed
-
- von Reyn CF, Horsburgh CR, Olivier KN, Barnes PF, Waddell R, Warren C, Tvaroha S, Jaeger AS, Lein AD, Alexander LN, Weber DJ, Tosteson AN. Skin test reactions to Mycobacterium tuberculosis purified protein derivative and Mycobacterium avium sensitin among health care workers and medical students in the United States. Int J Tuberc Lung Dis. 2001;5:1122–1128. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

