Membrane binding of an acyl-lactoferricin B antimicrobial peptide from solid-state NMR experiments and molecular dynamics simulations
- PMID: 21477580
- PMCID: PMC3124939
- DOI: 10.1016/j.bbamem.2011.03.017
Membrane binding of an acyl-lactoferricin B antimicrobial peptide from solid-state NMR experiments and molecular dynamics simulations
Abstract
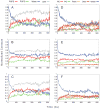
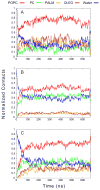
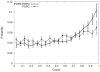
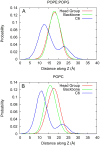
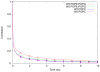
One approach to the growing health problem of antibiotic resistant bacteria is the development of antimicrobial peptides (AMPs) as alternative treatments. The mechanism by which these AMPs selectively attack the bacterial membrane is not well understood, but is believed to depend on differences in membrane lipid composition. N-acylation of the small amidated hexapeptide, RRWQWR-NH(2) (LfB6), derived from the 25 amino acid bovine lactoferricin (LfB25) can be an effective means to improve its antimicrobial properties. Here, we investigate the interactions of C6-LfB6, N-acylated with a 6 carbon fatty acid, with model lipid bilayers with two distinct compositions: 3:1 POPE:POPG (negatively charged) and POPC (zwitterionic). Results from solid-state (2)H and (31)P NMR experiments are compared with those from an ensemble of all-atom molecular dynamic simulations running in aggregate more than 8.6ms. (2)H NMR spectra reveal no change in the lipid acyl chain order when C6-LfB6 is bound to the negatively charged membrane and only a slight decrease in order when it is bound to the zwitterionic membrane. (31)P NMR spectra show no significant perturbation of the phosphate head groups of either lipid system in the presence of C6-LfB6. Molecular dynamic simulations show that for the negatively charged membrane, the peptide's arginines drive the initial association with the membrane, followed by attachment of the tryptophans at the membrane-water interface, and finally by the insertion of the C6 tails deep into the bilayer. In contrast, the C6 tail leads the association with the zwitterionic membrane, with the tryptophans and arginines associating with the membrane-water interface in roughly the same amount of time. We find similar patterns in the order parameters from our simulations. Moreover, we find in the simulations that the C6 tail can insert 1-2Å more deeply into the zwitterionic membrane and can exist in a wider range of angles than in the negatively charged membrane. We propose this is due to the larger area per lipid in the zwitterionic membrane, which provides more space for the C6 to insert and assume different orientations.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures

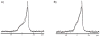




Similar articles
-
Coupling molecular dynamics simulations with experiments for the rational design of indolicidin-analogous antimicrobial peptides.J Mol Biol. 2009 Sep 25;392(3):837-54. doi: 10.1016/j.jmb.2009.06.071. Epub 2009 Jul 2. J Mol Biol. 2009. PMID: 19576903
-
Lipid interactions of acylated tryptophan-methylated lactoferricin peptides by solid-state NMR.J Pept Sci. 2008 Oct;14(10):1103-10. doi: 10.1002/psc.1047. J Pept Sci. 2008. PMID: 18523968
-
Solid-state nuclear magnetic resonance relaxation studies of the interaction mechanism of antimicrobial peptides with phospholipid bilayer membranes.Biochemistry. 2005 Aug 2;44(30):10208-17. doi: 10.1021/bi050730p. Biochemistry. 2005. PMID: 16042398
-
Structure and dynamics of phospholipids in membranes elucidated by combined use of NMR and vibrational spectroscopies.Biochim Biophys Acta Biomembr. 2020 Sep 1;1862(9):183352. doi: 10.1016/j.bbamem.2020.183352. Epub 2020 May 11. Biochim Biophys Acta Biomembr. 2020. PMID: 32407775 Review.
-
Towards a structure-function analysis of bovine lactoferricin and related tryptophan- and arginine-containing peptides.Biochem Cell Biol. 2002;80(1):49-63. doi: 10.1139/o01-213. Biochem Cell Biol. 2002. PMID: 11908643 Review.
Cited by
-
Design and Evaluation of Short Bovine Lactoferrin-Derived Antimicrobial Peptides against Multidrug-Resistant Enterococcus faecium.Antibiotics (Basel). 2022 Aug 10;11(8):1085. doi: 10.3390/antibiotics11081085. Antibiotics (Basel). 2022. PMID: 36009954 Free PMC article.
-
Effect of drug amlodipine on the charged lipid bilayer cell membranes DMPS and DMPS + DMPC: a molecular dynamics simulation study.Eur Biophys J. 2018 Dec;47(8):939-950. doi: 10.1007/s00249-018-1317-z. Epub 2018 Jul 3. Eur Biophys J. 2018. PMID: 29971510
-
Synergistic applications of MD and NMR for the study of biological systems.J Biomed Biotechnol. 2012;2012:254208. doi: 10.1155/2012/254208. Epub 2012 Jan 26. J Biomed Biotechnol. 2012. PMID: 22319241 Free PMC article. Review.
-
Thermodynamics of antimicrobial lipopeptide binding to membranes: origins of affinity and selectivity.Biophys J. 2014 Oct 21;107(8):1862-1872. doi: 10.1016/j.bpj.2014.08.026. Biophys J. 2014. PMID: 25418167 Free PMC article.
-
N-myristoylation of Antimicrobial Peptide CM4 Enhances Its Anticancer Activity by Interacting With Cell Membrane and Targeting Mitochondria in Breast Cancer Cells.Front Pharmacol. 2018 Nov 13;9:1297. doi: 10.3389/fphar.2018.01297. eCollection 2018. Front Pharmacol. 2018. PMID: 30483133 Free PMC article.
References
-
- Tomita M, Takase M, Bellamy W, Shimamura S. A review: the active peptide of lactoferrin. Acta Paediatr Jpn. 1994;36:585–91. - PubMed
-
- Boman HG. Peptide antibiotics and their role in innate immunity. Annu Rev Immunol. 1995;13:61–92. - PubMed
-
- Hancock R. Cationic peptides: effectors in innate immunity and novel antimicrobials. The Lancet infectious diseases. 2001;1:156–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

