An open-label trial of rituximab therapy in pulmonary alveolar proteinosis
- PMID: 21478218
- PMCID: PMC3874725
- DOI: 10.1183/09031936.00197710
An open-label trial of rituximab therapy in pulmonary alveolar proteinosis
Abstract
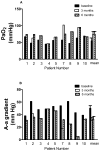
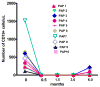
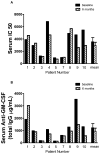
Rituximab, a monoclonal antibody directed against the B-lymphocyte antigen CD20, has shown promise in several autoimmune disorders. Pulmonary alveolar proteinosis (PAP) is an autoimmune disorder characterised by autoantibodies to granulocyte-macrophage colony-stimulating factor (GM-CSF). An open-label, proof-of-concept phase II clinical trial was conducted in 10 PAP patients. The intervention consisted of two intravenous infusions of rituximab (1,000 mg) 15 days apart. Bronchoalveolar lavage (BAL) fluid and peripheral blood samples were collected. The primary outcome was improvement in arterial blood oxygenation. Both arterial oxygen tension and alveolar-arterial oxygen tension difference in room air improved in seven out of the nine patients completing the study. Lung function and high-resolution computed tomography scans, which were secondary outcomes, also improved. Peripheral blood CD19+ B-lymphocytes decreased from mean ± sem 15 ± 2% to <0.05% (n = 10) 15 days post-therapy. This decrease persisted for 3 months in all patients; at 6 months, CD19+ B-cells were detected in four out of seven patients (5 ± 2%). Total anti-GM-CSF immunoglobulin (Ig)G levels from baseline to 6 months were decreased in BAL fluids (n = 8) but unchanged in sera (n = 9). In this PAP cohort: 1) rituximab was well-tolerated and effectively ameliorated lung disease; and 2) reduction in anti-GM-CSF IgG levels in the lung correlated with disease changes, suggesting that disease pathogenesis is related to autoantibody levels in the target organ.
Trial registration: ClinicalTrials.gov NCT00552461.
Figures




References
-
- Grillo-Lopez AJ, White CA, Varns C, Shen D, Wei A, McClure A, Dallaire BK. Overview of the clinical development of rituximab: first monoclonal antibody approved for the treatment of lymphoma. Semin Oncol. 1999;26:66–73. - PubMed
-
- Anolik J, Sanz I, Looney RJ. B cell depletion therapy in systemic lupus erythematosus. Curr Rheumatol Rep. 2003;5:350–356. - PubMed
-
- Edwards JC, Szczepanski L, Szechinski J, Filipowicz-Sosnowska A, Emery P, Close DR, Stevens RM, Shaw T. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med. 2004;350:2572–2581. - PubMed
-
- Cambridge G, Leandro MJ, Edwards JC, Ehrenstein MR, Salden M, Bodman-Smith M, Webster AD. Serologic changes following B lymphocyte depletion therapy for rheumatoid arthritis. Arthritis Rheum. 2003;48:2146–2154. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
