Long-term neuropathy after oxaliplatin treatment: challenging the dictum of reversibility
- PMID: 21478275
- PMCID: PMC3228192
- DOI: 10.1634/theoncologist.2010-0248
Long-term neuropathy after oxaliplatin treatment: challenging the dictum of reversibility
Abstract
Objectives: Oxaliplatin-induced neuropathy is a significant and dose-limiting toxicity that adversely affects quality of life. However, the long-term neurological sequelae have not been adequately described. The present study aimed to describe the natural history of oxaliplatin-induced neuropathy, using subjective and objective assessments.
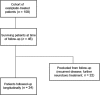
Methods: From a population of 108 oxaliplatin-treated patients referred for neurological assessment in 2002-2008, 52.2% of the surviving patient cohort (n = 24) was available for follow-up at a median of 25 months post-oxaliplatin. Patients underwent a protocol that incorporated clinical assessment scales, patient questionnaires, standard electrodiagnostic assessments, and novel nerve excitability studies to precisely assess nerve function.
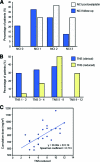
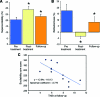
Results: At follow-up, 79.2% of patients reported residual neuropathic symptoms, with distal loss of pin-prick sensibility in 58.3% of patients and loss of vibration sensibility in 83.3% of patients. Symptom severity scores were significantly correlated with cumulative dose. There was no recovery of sensory action potential amplitudes in upper and lower limbs, consistent with persistent axonal sensory neuropathy. Sensory excitability parameters had not returned to baseline levels, suggesting persisting abnormalities in nerve function. The extent of excitability abnormalities during treatment was significantly correlated with clinical outcomes at follow-up.
Conclusions: These findings establish the persistence of subjective and objective deficits in oxaliplatin-treated patients post-oxaliplatin, suggesting that sensory neuropathy is a long-term outcome, thereby challenging the literature on the reversibility of oxaliplatin-induced neuropathy.
Conflict of interest statement
Section Editor
Section Editor
Reviewers “A” and “B” disclose no financial relationships.
The content of this article has been reviewed by independent peer reviewers to ensure that it is balanced, objective, and free from commercial bias. On the basis of disclosed information, all conflicts of interest have been resolved.
Figures

References
-
- André T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343–2351. - PubMed
-
- André T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27:3109–3116. - PubMed
-
- de Gramont A, Figer A, Seymour M, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–2947. - PubMed
-
- Gamelin E, Gamelin L, Bossi L, et al. Clinical aspects and molecular basis of oxaliplatin neurotoxicity: Current management and development of preventive measures. Semin Oncol. 2002;29(5 suppl 15):21–33. - PubMed
-
- Grothey A. Oxaliplatin-safety profile: Neurotoxicity. Semin Oncol. 2003;30(4 suppl 15):5–13. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources

