Meiotic homologue alignment and its quality surveillance are controlled by mouse HORMAD1
- PMID: 21478856
- PMCID: PMC3087846
- DOI: 10.1038/ncb2213
Meiotic homologue alignment and its quality surveillance are controlled by mouse HORMAD1
Abstract
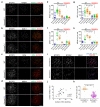
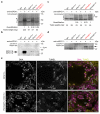
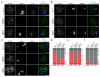
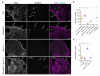
Meiotic crossover formation between homologous chromosomes (homologues) entails DNA double-strand break (DSB) formation, homology search using DSB ends, and synaptonemal-complex formation coupled with DSB repair. Meiotic progression must be prevented until DSB repair and homologue alignment are completed, to avoid the formation of aneuploid gametes. Here we show that mouse HORMAD1 ensures that sufficient numbers of processed DSBs are available for successful homology search. HORMAD1 is needed for normal synaptonemal-complex formation and for the efficient recruitment of ATR checkpoint kinase activity to unsynapsed chromatin. The latter phenomenon was proposed to be important in meiotic prophase checkpoints in both sexes. Consistent with this hypothesis, HORMAD1 is essential for the elimination of synaptonemal-complex-defective oocytes. Synaptonemal-complex formation results in HORMAD1 depletion from chromosome axes. Thus, we propose that the synaptonemal complex and HORMAD1 are key components of a negative feedback loop that coordinates meiotic progression with homologue alignment: HORMAD1 promotes homologue alignment and synaptonemal-complex formation, and synaptonemal complexes downregulate HORMAD1 function, thereby permitting progression past meiotic prophase checkpoints.
Figures



References
-
- Baudat F, de Massy B. Regulating double-stranded DNA break repair towards crossover or non-crossover during mammalian meiosis. Chromosome Res. 2007;15:565–577. - PubMed
-
- Hunter N. Meiotic recombination in Molecular genetics of recombination. Springer-Verlag; Berlin Heidelberg: 2007.
-
- Keeney S, Giroux CN, Kleckner N. Meiosis-specific DNA double-strand breaks are catalyzed by Spo11, a member of a widely conserved protein family. Cell. 1997;88:375–384. - PubMed
-
- Baudat F, Manova K, Yuen JP, Jasin M, Keeney S. Chromosome synapsis defects and sexually dimorphic meiotic progression in mice lacking Spo11. Mol Cell. 2000;6:989–998. - PubMed
-
- Romanienko PJ, Camerini-Otero RD. The mouse Spo11 gene is required for meiotic chromosome synapsis. Mol Cell. 2000;6:975–987. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

