Structural basis for the role of inhibition in facilitating adult brain plasticity
- PMID: 21478885
- PMCID: PMC3083474
- DOI: 10.1038/nn.2799
Structural basis for the role of inhibition in facilitating adult brain plasticity
Abstract
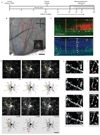
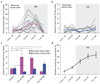
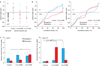
Although inhibition has been implicated in mediating plasticity in the adult brain, the underlying mechanism remains unclear. Here we present a structural mechanism for the role of inhibition in experience-dependent plasticity. Using chronic in vivo two-photon microscopy in the mouse neocortex, we show that experience drives structural remodeling of superficial layer 2/3 interneurons in an input- and circuit-specific manner, with up to 16% of branch tips undergoing remodeling. Visual deprivation initially induces dendritic branch retractions, and this is accompanied by a loss of inhibitory inputs onto neighboring pyramidal cells. The resulting decrease in inhibitory tone, also achievable pharmacologically using the antidepressant fluoxetine, provides a permissive environment for further structural adaptation, including addition of new synapse-bearing branch tips. Our findings suggest that therapeutic approaches that reduce inhibition, when combined with an instructive stimulus, could facilitate restructuring of mature circuits impaired by damage or disease, improving function and perhaps enhancing cognitive abilities.
Figures




References
-
- Fernandez F, et al. Pharmacotherapy for cognitive impairment in a mouse model of Down syndrome. Nat Neurosci. 2007;10:411–413. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

