Influence of caloric restriction on constitutive expression of NF-κB in an experimental mouse astrocytoma
- PMID: 21479220
- PMCID: PMC3068150
- DOI: 10.1371/journal.pone.0018085
Influence of caloric restriction on constitutive expression of NF-κB in an experimental mouse astrocytoma
Abstract
Background: Many of the current standard therapies employed for the management of primary malignant brain cancers are largely viewed as palliative, ultimately because these conventional strategies have been shown, in many instances, to decrease patient quality of life while only offering a modest increase in the length of survival. We propose that caloric restriction (CR) is an alternative metabolic therapy for brain cancer management that will not only improve survival but also reduce the morbidity associated with disease. Although we have shown that CR manages tumor growth and improves survival through multiple molecular and biochemical mechanisms, little information is known about the role that CR plays in modulating inflammation in brain tumor tissue.
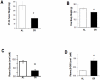
Methodology/principal findings: Phosphorylation and activation of nuclear factor κB (NF-κB) results in the transactivation of many genes including those encoding cycloxygenase-2 (COX-2) and allograft inflammatory factor-1 (AIF-1), both of which are proteins that are primarily expressed by inflammatory and malignant cancer cells. COX-2 has been shown to enhance inflammation and promote tumor cell survival in both in vitro and in vivo studies. In the current report, we demonstrate that the p65 subunit of NF-κB was expressed constitutively in the CT-2A tumor compared with contra-lateral normal brain tissue, and we also show that CR reduces (i) the phosphorylation and degree of transcriptional activation of the NF-κB-dependent genes COX-2 and AIF-1 in tumor tissue, as well as (ii) the expression of proinflammatory markers lying downstream of NF-κB in the CT-2A malignant mouse astrocytoma, [e.g. macrophage inflammatory protein-2 (MIP-2)]. On the whole, our date indicate that the NF-κB inflammatory pathway is constitutively activated in the CT-2A astrocytoma and that CR targets this pathway and inflammation.
Conclusion: CR could be effective in reducing malignant brain tumor growth in part by inhibiting inflammation in the primary brain tumor.
Conflict of interest statement
Figures





References
-
- Fisher PG, Buffler PA. Malignant gliomas in 2005: where to GO from here? Jama. 2005;293:615–617. - PubMed
-
- Nicholson HS, Kretschmar CS, Krailo M, Bernstein M, Kadota R, et al. Phase 2 study of temozolomide in children and adolescents with recurrent central nervous system tumors: a report from the Children's Oncology Group. Cancer. 2007;110:1542–1550. - PubMed
-
- Lowry JK, Snyder JJ, Lowry PW. Brain tumors in the elderly: recent trends in a Minnesota cohort study. Arch Neurol. 1998;55:922–928. - PubMed
-
- Smith JS, Jenkins RB. Genetic alterations in adult diffuse glioma: occurrence, significance, and prognostic implications. Front Biosci. 2000;5:D213–231. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

