Fibrinogen-γ proteolysis and solubility dynamics during apoptotic mouse liver injury: heparin prevents and treats liver damage
- PMID: 21480334
- PMCID: PMC3079287
- DOI: 10.1002/hep.24203
Fibrinogen-γ proteolysis and solubility dynamics during apoptotic mouse liver injury: heparin prevents and treats liver damage
Abstract
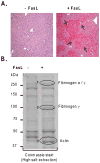
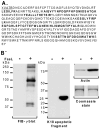
Fas ligand (FasL)-mediated hepatocyte apoptosis occurs in the context of acute liver injury that can be accompanied by intravascular coagulation (IC). We tested the hypothesis that analysis of selected protein fractions from livers undergoing apoptosis will shed light on mechanisms that are involved in liver injury that might be amenable to intervention. Proteomic analysis of the major insoluble liver proteins after FasL exposure for 4-5 hours identified fibrinogen-γ (FIB-γ) dimers and FIB-γ-containing high molecular mass complexes among the major insoluble proteins visible via Coomassie blue staining. Presence of the FIB-γ-containing products was confirmed using FIB-γ-specific antibodies. The FIB-γ-containing products partition selectively and quantitatively into the liver parenchyma after inducing apoptosis. Similar formation of FIB-γ products occurs after acetaminophen administration. The observed intrahepatic IC raised the possibility that heparin therapy may ameliorate FasL-mediated liver injury. Notably, heparin administration in mice 4 hours before or up to 2 hours after FasL injection resulted in a dramatic reduction of liver injury-including liver hemorrhage, serum alanine aminotransferase, caspase activation, and liver apoptosis-compared with heparin-untreated mice. Heparin did not directly interfere with FasL-induced apoptosis in isolated hepatocytes, and heparin-treated mice survived the FasL-induced liver injury longer compared with heparin-untreated animals. There was a sharp, near-simultaneous rise in FasL-induced intrahepatic apoptosis and coagulation, with IC remaining stable while apoptosis continued to increase.
Conclusion: Formation of FIB-γ dimers and their high molecular mass products are readily detectable within the liver during mouse apoptotic liver injury. Heparin provides a potential therapeutic modality, because it not only prevents extensive FasL-related liver injury but also limits the extent of injury if given at early stages of injury exposure.
American Association for the Study of Liver Diseases.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
