Molecular modulation of prefrontal cortex: rational development of treatments for psychiatric disorders
- PMID: 21480691
- PMCID: PMC3109197
- DOI: 10.1037/a0023165
Molecular modulation of prefrontal cortex: rational development of treatments for psychiatric disorders
Abstract
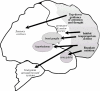
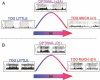
Dysfunction of the prefrontal cortex (PFC) is a central feature of many psychiatric disorders, such as attention deficit hyperactivity disorder (ADHD), posttraumatic stress disorder (PTSD), schizophrenia, and bipolar disorder. Thus, understanding molecular influences on PFC function through basic research in animals is essential to rational drug development. In this review, we discuss the molecular signaling events initiated by norepinephrine and dopamine that strengthen working memory function mediated by the dorsolateral PFC under optimal conditions, and weaken working memory function during uncontrollable stress. We also discuss how these intracellular mechanisms can be compromised in psychiatric disorders, and how novel treatments based on these findings may restore a molecular environment conducive to PFC regulation of behavior, thought and emotion. Examples of successful translation from animals to humans include guanfacine for the treatment of ADHD and related PFC disorders, and prazosin for the treatment of PTSD.
Figures


References
-
- Aoki C, Go CG, Venkatesan C, Kurose H. Perikaryal and synaptic localization of alpha 2A-adrenergic receptor-like immunoreactivity. Brain Research. 1994;650(2):181–204. - PubMed
-
- Aoki C, Venkatesan C, Kurose H. Noradrenergic modulation of the prefrontal cortex as revealed by electron microscopic immunocytochemistry. Advances in Pharmacology (San Diego, Calif.) 1998;42:777–780. - PubMed
-
- Arnsten AFT, Castellanos FX. Neurobiology of attention regulation and its disorders. In: Martin A, Scahill L, Charney D, Leckman J, editors. Textbook of child and adolescent pharmacology. Oxford University Press; New York, NY: 2002. pp. 99–109.
-
- Arnsten AFT, Manji HK. Mania: A rational neurobiology. Future Neurology. 2008;3(2):125–131.
-
- Arnsten AFT, Vijayraghavan S, Wang M, Gamo NJ, Paspalas CD. Dopamine's influence on prefrontal cortical cognition: Actions and circuits in behaving primates. In: Iversen L, Iversen S, Dunnett S, Bjorklund A, editors. Dopamine handbook. 1st ed. Oxford University Press; New York, NY: 2009. pp. 230–248.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

