On the relationship between block of the cardiac Na⁺ channel and drug-induced prolongation of the QRS complex
- PMID: 21480866
- PMCID: PMC3174407
- DOI: 10.1111/j.1476-5381.2011.01415.x
On the relationship between block of the cardiac Na⁺ channel and drug-induced prolongation of the QRS complex
Abstract
Background and purpose: Inhibition of the human cardiac Na(+) channel (hNa(v) 1.5) can prolong the QRS complex and has been associated with increased mortality in patients with underlying cardiovascular disease. The safety implications of blocking hNa(v) 1.5 channels suggest the need to test for this activity early in drug discovery in order to design out any potential liability. However, interpretation of hNa(v) 1.5 blocking potency requires knowledge of how hNa(v) 1.5 block translates into prolongation of the QRS complex.
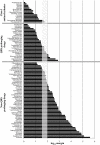
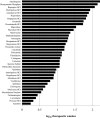
Experimental approach: We tested Class I anti-arrhythmics, other known QRS prolonging drugs and drugs not reported to prolong the QRS complex. Their block of hNa(v) 1.5 channels (as IC(50) values) was measured in an automated electrophysiology-based assay. These IC(50) values were compared with published reports of the corresponding unbound (free) plasma concentrations attained during clinical use (fC(max)) to provide an IC(50) : fC(max) ratio. KEY RESULTS For 42 Class I anti-arrhythmics and other QRS prolonging drugs, 67% had IC(50) : fC(max) ratios <30. For 55 non-QRS prolonging drugs tested, 72% had ratios >100. Finally, we determined the relationship between the IC(50) value and the free drug concentration associated with prolongation of the QRS complex in humans. For 37 drugs, QRS complex prolongation was observed at free plasma concentrations that were about 15-fold lower than the corresponding IC(50) at hNa(v) 1.5 channels.
Conclusions and implications: A margin of 30- to 100-fold between hNa(v) 1.5 IC(50) and fC(max) appears to confer an acceptable degree of safety from QRS prolongation. QRS prolongation occurs on average at free plasma levels 15-fold below the IC(50) at hNa(v) 1.5 channels.
Linked article: This article is commented on by Gintant et al., pp. 254-259 of this issue. To view this commentary visit http://dx.doi.org/10.1111/j.1476-5381.2011.01433.x.
© 2011 AstraZeneca. British Journal of Pharmacology © 2011 The British Pharmacological Society.
Figures


References
-
- Adesanya CO, Yousuf KA, Co C, Gaur S, Ahmed S, Pothoulakis A, et al. Is wider worse? QRS duration predicts cardiac mortality in patients with right bundle branch block. Ann Noninvasive Electrocardiol. 2008;13:165–170. DOI: 10.1111/j.1542-474X.2008.00216.x. - DOI - PMC - PubMed
-
- Anonymous. ICH S7A safety pharmacology studies for human pharmaceuticals. 2000. London, 16 November 2000. CPMP/ICH/539/00. ICH S7B. CPMP/ICH/539/00.
-
- Anonymous. The clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs. 2005a. ICH E14. CHMP/ICH/2/04. - PubMed
-
- Anonymous. The nonclinical evaluation of the potential for delayed ventricular repolarisation (QT interval prolongation) by human pharmaceuticals. 2005b. ICH S7B. CHMP/ICH/423/02. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

