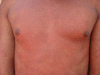
Hypersensitivity reactions to HIV therapy
- PMID: 21480946
- PMCID: PMC3093072
- DOI: 10.1111/j.1365-2125.2010.03784.x
Hypersensitivity reactions to HIV therapy
Abstract
Many drugs used for the treatment of HIV disease (including the associated opportunistic infections) can cause drug hypersensitivity reactions, which vary in severity, clinical manifestations and frequency. These reactions are not only seen with the older compounds, but also with the newer more recently introduced drugs. The pathogenesis is unclear in most cases, but there is increasing evidence to support that many of these are mediated through a combination of immunologic and genetic factors through the major histocompatibility complex (MHC). Genetic predisposition to the occurrence of these allergic reactions has been shown for some of the drugs, notably abacavir hypersensitivity which is strongly associated with the class I MHC allele, HLA-B*5701. Testing before the prescription of abacavir has been shown to be of clinical utility, has resulted in a change in the drug label, is now recommended in clinical guidelines and is practiced in most Western countries. For most other drugs, however, there are no good methods of prevention, and clinical monitoring with appropriate (usually supportive and symptomatic) treatment is required. There is a need to undertake further research in this area to increase our understanding of the mechanisms, which may lead to better preventive strategies through the development of predictive genetic biomarkers or through guiding the design of drugs less likely to cause these types of adverse drug reactions.
© 2011 The Authors. British Journal of Clinical Pharmacology © 2011 The British Pharmacological Society.
Figures
References
-
- Breckenridge A. Pharmacology of drugs for HIV. Medicine. 2009;37:374–7.
-
- Hughes CA, Robinson L, Tseng A, MacArthur RD. New antiretroviral drugs: a review of the efficacy, safety, pharmacokinetics, and resistance profile of tipranavir, darunavir, etravirine, rilpivirine, maraviroc, and raltegravir. Expert Opin Pharmacother. 2009;10:2445–66. - PubMed
-
- Palella FJ, Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, Aschman DJ, Holmberg SD. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–60. - PubMed
-
- Seth D, Kamat D, Montejo J. DRESS syndrome: a practical approach for primary care practitioners. Clin Pediatr (Phila) 2008;47:947–52. - PubMed
-
- Wolf R, Orion E, Marcos B, Matz H. Life-threatening acute adverse cutaneous drug reactions. Clin Dermatol. 2005;23:171–81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials