Skin manifestations of drug allergy
- PMID: 21480947
- PMCID: PMC3093073
- DOI: 10.1111/j.1365-2125.2010.03703.x
Skin manifestations of drug allergy
Abstract
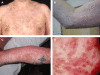
Cutaneous adverse drug reactions range from mild to severe and from those localized only to skin to those associated with systemic disease. It is important to distinguish features of cutaneous drug reactions which help classify the underlying mechanism and likely prognosis as both of these influence management decisions, some of which necessarily have to be taken rapidly. Severe cutaneous reactions are generally T cell-mediated, yet this immunological process is frequently poorly understood and principles for identification of the culprit drug are different to those of IgE mediated allergic reactions. Furthermore, intervention in severe skin manifestations of drug allergy is frequently necessary. However, a substantial literature reports on success or otherwise of glucocorticoids, cyclophsphamide, ciclosporin, intravenous immunoglobulin and anti-tumour necrosis factor therapy for the treatment of toxic epidermal necrolysis without clear consensus. As well as reviewing the recommended supportive measures and evidence base for interventions, this review aims to provide a mechanistic overview relating to a proposed clinical classification to assist the assessment and management of these complex patients.
© 2011 The Authors. British Journal of Clinical Pharmacology © 2011 The British Pharmacological Society.
Figures


Similar articles
-
Annular drug eruptions.Clin Dermatol. 2022 Sep-Oct;40(5):450-465. doi: 10.1016/j.clindermatol.2021.12.008. Epub 2021 Dec 31. Clin Dermatol. 2022. PMID: 34979270
-
[DRUG INDUCED EXANTHEMA AND SEVERE CUTANEOUS DRUG REACTIONS].Rev Prat. 2015 Sep;65(7):981-5. Rev Prat. 2015. PMID: 26619740 French.
-
Exanthems and drug reactions.Aust Fam Physician. 2011 Jul;40(7):486-9. Aust Fam Physician. 2011. PMID: 21743852 Review.
-
Adverse drug reactions and organ damage: The skin.Eur J Intern Med. 2016 Mar;28:17-24. doi: 10.1016/j.ejim.2015.11.017. Epub 2015 Dec 7. Eur J Intern Med. 2016. PMID: 26674736 Review.
-
[Adverse cutaneous reactions to drugs].Rev Med Inst Mex Seguro Soc. 2018 Jan-Feb;56(1):64-70. Rev Med Inst Mex Seguro Soc. 2018. PMID: 29368897 Review. Spanish.
Cited by
-
An Allergic Reaction in Contrast-enhanced Ultrasound Lymphography for Lymphovenous Bypass Surgery.Plast Reconstr Surg Glob Open. 2024 Jun 17;12(6):e5908. doi: 10.1097/GOX.0000000000005908. eCollection 2024 Jun. Plast Reconstr Surg Glob Open. 2024. PMID: 38911583 Free PMC article.
-
Generalized purpuric drug exanthem with hemorrhagic plaques following bendamustine chemotherapy in a patient with B-prolymphocytic leukemia.Int J Hematol. 2012 Mar;95(3):311-4. doi: 10.1007/s12185-012-1012-2. Int J Hematol. 2012. PMID: 22290027
-
Allergy, pseudo-allergy and non-allergy.Br J Clin Pharmacol. 2011 May;71(5):637-8. doi: 10.1111/j.1365-2125.2011.03976.x. Br J Clin Pharmacol. 2011. PMID: 21480945 Free PMC article. No abstract available.
-
Rosacea-like facial rash related to metformin administration in a young woman.BMC Pharmacol Toxicol. 2014 Feb 8;15:3. doi: 10.1186/2050-6511-15-3. BMC Pharmacol Toxicol. 2014. PMID: 24507578 Free PMC article.
-
Diagnosing and managing drug allergy.CMAJ. 2018 Apr 30;190(17):E532-E538. doi: 10.1503/cmaj.171315. CMAJ. 2018. PMID: 29712672 Free PMC article. Review. No abstract available.
References
-
- Bigby M, Jick S, Jick H, Arndt K. Drug-induced cutaneous reactions. A report from the Boston Collaborative Drug Surveillance Program on 15,438 consecutive inpatients, 1975 to 1982. JAMA. 1986;256:3358–63. - PubMed
-
- Hunziker T, Kunzi UP, Braunschweig S, Zehnder D, Hoigne R. Comprehensive hospital drug monitoring (CHDM): adverse skin reactions, a 20-year survey. Allergy. 1997;52:388–93. - PubMed
-
- Fiszenson-Albala F, Auzerie V, Mahe E, Farinotti R, Durand-Stocco C, Crickx B, Descamps V. A 6-month prospective survey of cutaneous drug reactions in a hospital setting. Br J Dermatol. 2003;149:1018–22. - PubMed
-
- Merk HF, Hertl M. Immunologic mechanisms of cutaneous drug reactions. Semin Cutan Med Surg. 1996;15:228–35. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

