Isolating and defining cells to engineer human blood vessels
- PMID: 21481038
- PMCID: PMC3387928
- DOI: 10.1111/j.1365-2184.2010.00719.x
Isolating and defining cells to engineer human blood vessels
Abstract
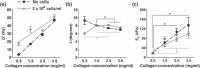
A great deal of attention has been recently focused on understanding the role that bone marrow-derived putative endothelial progenitor cells (EPC) may play in the process of neoangiogenesis. However, recent data indicate that many of the putative EPC populations are comprised of various haematopoietic cell subsets with proangiogenic activity, but these marrow-derived putative EPC fail to display vasculogenic activity. Rather, this property is reserved for a rare population of circulating viable endothelial cells with colony-forming cell (ECFC) ability. Indeed, human ECFC possess clonal proliferative potential, display endothelial and not haematopoietic cell surface antigens, and display in vivo vasculogenic activity when suspended in an extracellular matrix and implanted into immunodeficient mice. Furthermore, human vessels derived became integrated into the murine circulatory system and eventually were remodelled into arterial and venous vessels. Identification of this population now permits determination of optimal type I collagen matrix microenvironment into which the cells should be embedded and delivered to accelerate and even pattern number and size of blood vessels formed, in vivo. Indeed, altering physical properties of ECFC-collagen matrix implants changed numerous parameters of human blood vessel formation, in host mice. These recent discoveries may permit a strategy for patterning vascular beds for eventual tissue and organ regeneration.
© 2011 Blackwell Publishing Ltd.
Figures




References
-
- Otten J, Schultze A, Schafhausen P, Otterstetter S, Dierlamm J, Bokemeyer C et al. (2008) Blood outgrowth endothelial cells from chronic myeloid leukaemia patients are BCR/ABL1 negative. Br. J. Haematol. 142, 115–118. - PubMed
-
- Piaggio G, Rosti V, Corselli M, Bertolotti F, Bergamaschi G, Pozzi S et al. (2009) Endothelial colony‐forming cells from patients with chronic myeloproliferative disorders lack the disease‐specific molecular clonality marker. Blood 114, 3127–3130. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

