Oncostatin M promotes STAT3 activation, VEGF production, and invasion in osteosarcoma cell lines
- PMID: 21481226
- PMCID: PMC3079692
- DOI: 10.1186/1471-2407-11-125
Oncostatin M promotes STAT3 activation, VEGF production, and invasion in osteosarcoma cell lines
Abstract
Background: We have previously demonstrated that both canine and human OSA cell lines, as well as 8 fresh canine OSA tumor samples, exhibit constitutive phosphorylation of STAT3, and that this correlates with enhanced expression of matrix metalloproteinase-2 (MMP2). While multiple signal transduction pathways can result in phosphorylation of STAT3, stimulation of the cytokine receptor gp130 through either IL-6 or Oncostatin M (OSM) is the most common mechanism through which STAT3 is activated. The purpose of this study was to evaluate the role of IL-6 and OSM stimulation on both canine and human OSA cell lines to begin to determine the role of these cytokines in the biology of OSA.
Methods: RT-PCR and Western blotting were used to interrogate the consequences of OSM and IL-6 stimulation of OSA cell lines. OSA cells were stimulated with OSM and/or hepatocyte growth factor (HGF) and the effects on MMP2 activity (gel zymography), proliferation (CyQUANT), invasion (Matrigel transwell assay), and VEGF production (Western blotting, ELISA) were assessed. The small molecule STAT3 inhibitor LLL3 was used to investigate the impact of STAT3 inhibition following OSM stimulation of OSA cells.
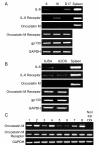
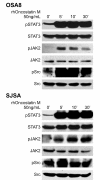
Results: Our data demonstrate that the OSM receptor (OSMR), but not IL-6 or its receptor, is expressed by all human and canine OSA cell lines and canine OSA tumor samples; additionally, OSM expression was noted in all tumor samples. Treatment of OSA cell lines with OSM induced phosphorylation of STAT3, Src, and JAK2. OSM stimulation also resulted in a dose dependent increase in MMP2 activity and VEGF expression that was markedly reduced following treatment with the small molecule STAT3 inhibitor LLL3. Lastly, OSM stimulation of OSA cell lines enhanced invasion through Matrigel, particularly in the presence of rhHGF. In contrast, both OSM and HGF stimulation of OSA cell lines did not alter their proliferative capacity.
Conclusions: These data indicate OSM stimulation of human and canine OSA cells induces STAT3 activation, thereby enhancing the expression/activation of MMP2 and VEGF, ultimately promoting invasive behavior and tumor angiogenesis. As such, OSM and its receptor may represent a novel target for therapeutic intervention in OSA.
Figures




References
-
- Withrow SJ, Powers BE, Straw RC, Wilkins RM. Comparative aspects of osteosarcoma. Dog versus man. Clin Orthop. 1991;270:159–168. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

