Onset of efficacy with acute long-acting injectable paliperidone palmitate treatment in markedly to severely ill patients with schizophrenia: post hoc analysis of a randomized, double-blind clinical trial
- PMID: 21481243
- PMCID: PMC3082227
- DOI: 10.1186/1744-859X-10-12
Onset of efficacy with acute long-acting injectable paliperidone palmitate treatment in markedly to severely ill patients with schizophrenia: post hoc analysis of a randomized, double-blind clinical trial
Abstract
Background: This post hoc analysis (trial registration: ClinicalTrials.gov NCT00590577) assessed onset of efficacy and tolerability of acute treatment with once-monthly paliperidone palmitate (PP), a long-acting atypical antipsychotic initiated by day 1 and day 8 injections, in a markedly to severely ill schizophrenia population.
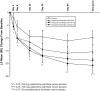
Methods: Subjects entering the 13-week, double-blind trial were randomized to PP (39, 156, or 234 mg [25, 100, and 150 mg eq of paliperidone, respectively]) or placebo. This subgroup analysis included those with a baseline Clinical Global Impressions-Severity (CGI-S) score indicating marked to severe illness. PP subjects received a 234-mg day 1 injection (deltoid), followed by their assigned dose on day 8 and monthly thereafter (deltoid or gluteal). Thus, data for PP groups were pooled for days 4 and 8. Measures included Positive and Negative Syndrome Scale (PANSS), CGI-S, Personal and Social Performance (PSP), and adverse events (AEs). Analysis of covariance (ANCOVA) and last-observation-carried-forward (LOCF) methodologies, without multiplicity adjustments, were used to assess changes in continuous measures. Onset of efficacy was defined as the first time point a treatment group showed significant PANSS improvement (assessed days 4, 8, 22, 36, 64, and 92) versus placebo, which was maintained through end point.
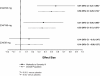
Results: A total of 312 subjects met inclusion criterion for this subgroup analysis. After the day 1 injection, mean PANSS total scores improved significantly with PP (all received 234 mg) versus placebo at day 4 (P = 0.012) and day 8 (P = 0.007). After the day 8 injection, a significant PANSS improvement persisted at all subsequent time points in the 234-mg group versus placebo (P < 0.05). PANSS improvements were greater from day 36 through end point in the 156-mg group (P < 0.05) and only at end point in the 39-mg group (P < 0.05). CGI-S and PSP scores improved significantly in the 234-mg and 156-mg PP groups versus placebo at end point (P < 0.05 for both, respectively); improvement in the 39-mg group was not significant. The most common AEs for PP-treated subjects (≥10%, any treatment group) were headache, insomnia, schizophrenia exacerbation, injection site pain, and agitation.
Conclusions: In this markedly to severely ill population, acute treatment with 234 mg PP improved psychotic symptoms compared with placebo by day 4. After subsequent injections, observed improvements are suggestive of a dose-dependent effect. No unexpected tolerability findings were noted.
Figures


References
-
- Lehman AF, Lieberman JA, Dixon LB, McGlashan TH, Miller AL, Perkins DO, Kreyenbuhl J. Practice guideline for the treatment of patients with schizophrenia. Am J Psychiatry. 2004;161(Suppl):1–56. - PubMed
-
- Kinon BJ, Chen L, Ascher-Svanum H, Stauffer VL, Kollack-Walker S, Zhou W, Kapur S, Kane JM. Early response to antipsychotic drug therapy as a clinical marker of subsequent response in the treatment of schizophrenia. Neuropsychopharmacology. 2010;35:581–590. doi: 10.1038/npp.2009.164. - DOI - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

