Neuroanatomical evidence for reciprocal regulation of the corticotrophin-releasing factor and oxytocin systems in the hypothalamus and the bed nucleus of the stria terminalis of the rat: Implications for balancing stress and affect
- PMID: 21481539
- PMCID: PMC3142325
- DOI: 10.1016/j.psyneuen.2011.03.003
Neuroanatomical evidence for reciprocal regulation of the corticotrophin-releasing factor and oxytocin systems in the hypothalamus and the bed nucleus of the stria terminalis of the rat: Implications for balancing stress and affect
Abstract
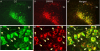
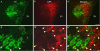
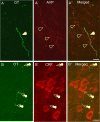
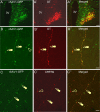
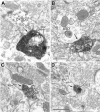
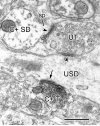
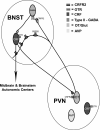
Activation of corticotrophin releasing factor (CRF) neurons in the paraventricular nucleus of the hypothalamus (PVN) is necessary for establishing the classic endocrine response to stress, while activation of forebrain CRF neurons mediates affective components of the stress response. Previous studies have reported that mRNA for CRF2 receptor (CRFR2) is expressed in the bed nucleus of the stria terminalis (BNST) as well as hypothalamic nuclei, but little is known about the localization and cellular distribution of CRFR2 in these regions. Using immunofluorescence with confocal microscopy, as well as electron microscopy, we demonstrate that in the BNST CRFR2-immunoreactive fibers represent moderate to strong labeling on axons terminals. Dual-immunofluorescence demonstrated that CRFR2-fibers co-localize oxytocin (OT), but not arginine-vasopressin (AVP), and make perisomatic contacts with CRF neurons. Dual-immunofluorescence and single cell RT-PCR demonstrate that in the hypothalamus, CRFR2 immunoreactivity and mRNA are found in OT, but not in CRF or AVP-neurons. Furthermore, CRF neurons of the PVN and BNST express mRNA for the oxytocin receptor, while the majority of OT/CRFR2 neurons in the hypothalamus do not. Finally, using adenoviral-based anterograde tracing of PVN neurons, we show that OT/CRFR2-immunoreactive fibers observed in the BNST originate in the PVN. Our results strongly suggest that CRFR2 located on oxytocinergic neurons and axon terminals might regulate the release of this neuropeptide and hence might be a crucial part of potential feedback loop between the hypothalamic oxytocin system and the forebrain CRF system that could significantly impact affective and social behaviors, in particular during times of stress.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures


References
-
- Alheid GF. Extended amygdala and basal forebrain. Ann N Y Acad Sci. 2003;985:185–205. - PubMed
-
- Amico JA, Mantella RC, Vollmer RR, Li X. Anxiety and stress responses in female oxytocin deficient mice. J Neuroendocrinol. 2004;16:319–324. - PubMed
-
- Arima H, Aguilera G. Vasopressin and oxytocin neurones of hypothalamic supraoptic and paraventricular nuclei co-express mRNA for Type-1 and Type-2 corticotropin-releasing hormone receptors. J Neuroendocrinol. 2000;12:833–842. - PubMed
-
- Arluison M, Vankova M, Cesselin F, Leviel V. Origin of some enkephalin-containing afferents to the ventro-medial region of the globus pallidus in the rat. Brain Res Bull. 1990;25:25–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

