DNA polymerases provide a canon of strategies for translesion synthesis past oxidatively generated lesions
- PMID: 21482102
- PMCID: PMC3112272
- DOI: 10.1016/j.sbi.2011.03.008
DNA polymerases provide a canon of strategies for translesion synthesis past oxidatively generated lesions
Abstract
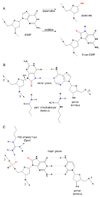
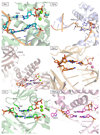
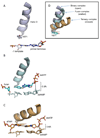
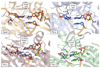
Deducing the structure of the DNA double helix in 1953 implied the mode of its replication: Watson-Crick (WC) base pairing might instruct an enzyme, now known as the DNA polymerase, during the synthesis of a daughter stand complementary to a single strand of the parental double helix. What has become increasingly clear in the last 60 years, however, is that adducted and oxidatively generated DNA bases are ubiquitous in physiological DNA, and all organisms conserve multiple DNA polymerases specialized for DNA synthesis opposite these damaged templates. Here, we review recent crystal structures depicting replicative and bypass DNA polymerases encountering two typical lesions arising from the oxidation of DNA: abasic sites, which block the replication fork, and the miscoding premutagenic lesion 7,8-dihydro-8-oxoguanine (8-oxoG).
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures
References
-
-
Loeb LA, Monnat RJ., Jr DNA polymerases and human disease. Nat Rev Genet. 2008;9:594–604. • This well-executed and thorough review describes the polymerases of the human genome inclusively, presenting some compelling figures to accompany the discussion.
-
-
- Bebenek K, Kunkel TA. Functions of DNA polymerases. Adv Protein Chem. 2004;69:137–165. - PubMed
-
- Lovett ST. Polymerase switching in DNA replication. Mol Cell. 2007;27:523–526. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

