Experience-dependent retinogeniculate synapse remodeling is abnormal in MeCP2-deficient mice
- PMID: 21482354
- PMCID: PMC3082316
- DOI: 10.1016/j.neuron.2011.03.001
Experience-dependent retinogeniculate synapse remodeling is abnormal in MeCP2-deficient mice
Abstract
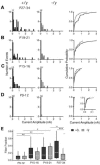
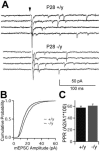
Mutations in MECP2 underlie the neurodevelopmental disorder Rett syndrome (RTT). One hallmark of RTT is relatively normal development followed by a later onset of symptoms. Growing evidence suggests an etiology of disrupted synaptic function, yet it is unclear how these abnormalities explain the clinical presentation of RTT. Here we investigate synapse maturation in Mecp2-deficient mice at a circuit with distinct developmental phases: the retinogeniculate synapse. We find that synapse development in mutants is comparable to that of wild-type littermates between postnatal days 9 and 21, indicating that initial phases of synapse formation, elimination, and strengthening are not significantly affected by MeCP2 absence. However, during the subsequent experience-dependent phase of synapse remodeling, the circuit becomes abnormal in mutants as retinal innervation of relay neurons increases and retinal inputs fail to strengthen further. Moreover, synaptic plasticity in response to visual deprivation is disrupted in mutants. These results suggest a crucial role for Mecp2 in experience-dependent refinement of synaptic circuits.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures



References
-
- Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23:185–188. - PubMed
-
- Armstrong DD, Deguchi K, Antallfy B. Survey of MeCP2 in the Rett syndrome and the non-Rett syndrome brain. J Child Neurol. 2003;18:683–687. - PubMed
-
- Asaka Y, Jugloff DG, Zhang L, Eubanks JH, Fitzsimonds RM. Hippocampal synaptic plasticity is impaired in the Mecp2-null mouse model of Rett syndrome. Neurobiol Dis. 2006;21:217–227. - PubMed
-
- Bader GG, Witt-Engerstrom I, Hagberg B. Neurophysiological findings in the Rett syndrome, II: Visual and auditory brainstem, middle and late evoked responses. Brain Dev. 1989;11:110–114. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

