Molecular basis for endothelial lumen formation and tubulogenesis during vasculogenesis and angiogenic sprouting
- PMID: 21482411
- PMCID: PMC3891664
- DOI: 10.1016/B978-0-12-386041-5.00003-0
Molecular basis for endothelial lumen formation and tubulogenesis during vasculogenesis and angiogenic sprouting
Abstract
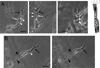
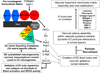
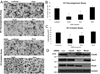
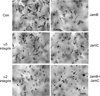
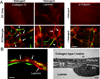
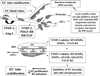
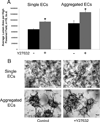
Many studies reveal a fundamental role for extracellular matrix-mediated signaling through integrins and Rho GTPases as well as matrix metalloproteinases (MMPs) in the molecular control of vascular tube morphogenesis in three-dimensional (3D) tissue environments. Recent work has defined an endothelial cell (EC) lumen signaling complex of proteins that controls these vascular morphogenic events. These findings reveal a signaling interdependence between Cdc42 and MT1-MMP to control the 3D matrix-specific process of EC tubulogenesis. The EC tube formation process results in the creation of a network of proteolytically generated vascular guidance tunnels in 3D matrices that are utilized to remodel EC-lined tubes through EC motility and could facilitate processes such as flow-induced remodeling and arteriovenous EC sorting and differentiation. Within vascular guidance tunnels, key dynamic interactions occur between ECs and pericytes to affect vessel remodeling, diameter, and vascular basement membrane matrix assembly, a fundamental process necessary for endothelial tube maturation and stabilization. Thus, the EC lumen and tube formation mechanism coordinates the concomitant establishment of a network of vascular tubes within tunnel spaces to allow for flow responsiveness, EC-mural cell interactions, and vascular extracellular matrix assembly to control the development of the functional microcirculation.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures







References
-
- Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol. 2007;8:464–478. - PubMed
-
- Alajati A, Laib AM, Weber H, Boos AM, Bartol A, Ikenberg K, Korff T, Zentgraf H, Obodozie C, Graeser R, Christian S, Finkenzeller G, Stark GB, Heroult M, Augustin HG. Spheroid-based engineering of a human vasculature in mice. Nat Methods. 2008;5:439–445. - PubMed
-
- Alavi A, Hood JD, Frausto R, Stupack DG, Cheresh DA. Role of Raf in vascular protection from distinct apoptotic stimuli. Science. 2003;301:94–96. - PubMed
-
- Aplin AC, Fogel E, Zorzi P, Nicosia RF. The aortic ring model of angiogenesis. Methods Enzymol. 2008;443:119–136. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

