A database of thermodynamic properties of the reactions of glycolysis, the tricarboxylic acid cycle, and the pentose phosphate pathway
- PMID: 21482578
- PMCID: PMC3077827
- DOI: 10.1093/database/bar005
A database of thermodynamic properties of the reactions of glycolysis, the tricarboxylic acid cycle, and the pentose phosphate pathway
Abstract
A database of thermodynamic properties is developed, which extends a previous database of glycolysis and tricarboxylic acid cycle by adding the reactions of the pentose phosphate pathway. The raw data and documented estimations of solution properties are made electronically available. The database is determined by estimation of a set of parameters representing species-level free energies of formation. The resulting calculations provide thermodynamic and network-based estimates of thermodynamic properties for six reactions of the pentose phosphate pathway for which estimates are not available in the preexisting literature. Optimized results are made available in ThermoML format. Because calculations depend on estimated hydrogen and metal cation dissociation constants, an uncertainty and sensitivity analysis is performed, revealing 23 critical dissociation constants to which the computed thermodynamic properties are particularly sensitive. DATABASE URL: http://www.biocoda.org/thermo
© The Author(s) 2011. Published by Oxford University Press.
Figures
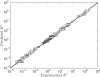
 versus experimental
versus experimental  . Model predicted apparent equilibrium constants under defined experimental conditions (T, I, [Mg2+], [Ca2+], [Na+], [K+] and pH) are plotted versus experimental measurements for all data used in the analysis. Data points in the pentose phosphate pathway are shown as filled squares.
. Model predicted apparent equilibrium constants under defined experimental conditions (T, I, [Mg2+], [Ca2+], [Na+], [K+] and pH) are plotted versus experimental measurements for all data used in the analysis. Data points in the pentose phosphate pathway are shown as filled squares.
 versus experimental
versus experimental  for the ribose-5-phosphate isomerase and ribuloase-phosphate 3-epimerase reactions. Open circles (GT-based
for the ribose-5-phosphate isomerase and ribuloase-phosphate 3-epimerase reactions. Open circles (GT-based  ) are computed based on the Goldberg's database (25); filled squares (optimized
) are computed based on the Goldberg's database (25); filled squares (optimized  ) are computed based on the optimized values of
) are computed based on the optimized values of  from Table 5.
from Table 5.
References
-
- Vojinović V, von Stockar U. Influence of uncertainties in pH, pMg, activity coefficients, metabolite concentrations, and other factors on the analysis of the thermodynamic feasibility of metabolic pathways. Biotechnol. Bioeng. 2009;103:780–795. - PubMed
-
- Mavrovouniotis ML. Identification of localized and distributed bottlenecks in metabolic pathways. In: Hunter L, Searls DB, Shavlik JW, editors. Proceedings of the First International Conference on Intelligent Systems for Molecular Biology. Bethesda, MD: AAAI Press; 1993. pp. 275–283. - PubMed
-
- Yang F, Beard DA. Thermodynamically based profiling of drug metabolism and drug-drug metabolic interactions: a case study of acetaminophen and ethanol toxic interaction. Biophys. Chem. 2006;120:121–134. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

