Tara up-regulates E-cadherin transcription by binding to the Trio RhoGEF and inhibiting Rac signaling
- PMID: 21482718
- PMCID: PMC3080255
- DOI: 10.1083/jcb.201009100
Tara up-regulates E-cadherin transcription by binding to the Trio RhoGEF and inhibiting Rac signaling
Abstract
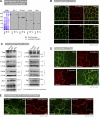
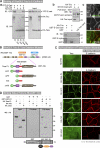
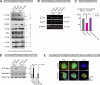
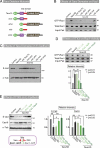
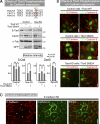
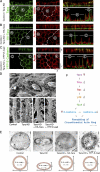
The spatiotemporal regulation of E-cadherin expression is important during body plan development and carcinogenesis. We found that Tara (Trio-associated repeat on actin) is enriched in cadherin-based adherens junctions (AJs), and its knockdown in MDCK cells (Tara-KD cells) significantly decreases the expression of E-cadherin. Tara-KD activates Rac1 through the Trio RhoGEF, which binds to E-cadherin and subsequently increases the phosphorylation of p38 and Tbx3, a transcriptional E-cadherin repressor. Accordingly, the decrease in E-cadherin expression is abrogated by ITX3 and SB203580 (specific inhibitors of Trio RhoGEF and p38MAPK, respectively), and by dephosphomimetic Tbx3. Despite the decreased E-cadherin expression, the Tara-KD cells do not undergo an epithelial-mesenchymal transition and remain as an epithelial cell sheet, presumably due to the concomitant up-regulation of cadherin-6. Tara-KD reduces the actin-belt density in the circumferential ring, and the cells form flattened cysts, suggesting that Tara functions to modulate epithelial cell sheet formation and integrity by up-regulating E-cadherin transcription.
Figures

Similar articles
-
N-cadherin signaling via Trio assembles adherens junctions to restrict endothelial permeability.J Cell Biol. 2019 Jan 7;218(1):299-316. doi: 10.1083/jcb.201802076. Epub 2018 Nov 21. J Cell Biol. 2019. PMID: 30463880 Free PMC article.
-
A local VE-cadherin and Trio-based signaling complex stabilizes endothelial junctions through Rac1.J Cell Sci. 2015 Aug 15;128(16):3041-54. doi: 10.1242/jcs.168674. Epub 2015 Jun 26. J Cell Sci. 2015. PMID: 26116572
-
FilGAP, a Rho-ROCK-regulated GAP for Rac, controls adherens junctions in MDCK cells.J Cell Sci. 2015 Jun 1;128(11):2047-56. doi: 10.1242/jcs.160192. Epub 2015 Apr 23. J Cell Sci. 2015. PMID: 25908853
-
Tara, a novel F-actin binding protein, associates with the Trio guanine nucleotide exchange factor and regulates actin cytoskeletal organization.J Cell Sci. 2001 Jan;114(Pt 2):389-99. doi: 10.1242/jcs.114.2.389. J Cell Sci. 2001. PMID: 11148140
-
Cadherin-mediated cell-cell interactions in normal and cancer cells.Tissue Barriers. 2017 Jul 3;5(3):e1356900. doi: 10.1080/21688370.2017.1356900. Epub 2017 Jul 20. Tissue Barriers. 2017. PMID: 28783415 Free PMC article. Review.
Cited by
-
The roles and regulation of TBX3 in development and disease.Gene. 2020 Feb 5;726:144223. doi: 10.1016/j.gene.2019.144223. Epub 2019 Oct 26. Gene. 2020. PMID: 31669645 Free PMC article. Review.
-
Monoclonal antibody for phosphorylated TRIO Y2681 that helps predict prognosis of post-operative colorectal cancer patients.BJC Rep. 2025 Jul 25;3(1):53. doi: 10.1038/s44276-025-00163-0. BJC Rep. 2025. PMID: 40715467 Free PMC article.
-
N-cadherin signaling via Trio assembles adherens junctions to restrict endothelial permeability.J Cell Biol. 2019 Jan 7;218(1):299-316. doi: 10.1083/jcb.201802076. Epub 2018 Nov 21. J Cell Biol. 2019. PMID: 30463880 Free PMC article.
-
The role of microtubules in the regulation of epithelial junctions.Tissue Barriers. 2018;6(3):1539596. doi: 10.1080/21688370.2018.1539596. Epub 2018 Nov 5. Tissue Barriers. 2018. PMID: 30395792 Free PMC article. Review.
-
Function and regulation of the Rho guanine nucleotide exchange factor Trio.Small GTPases. 2014;5:e29769. doi: 10.4161/sgtp.29769. Epub 2014 Jul 2. Small GTPases. 2014. PMID: 24987837 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

