Defective nucleotide excision repair with normal centrosome structures and functions in the absence of all vertebrate centrins
- PMID: 21482720
- PMCID: PMC3080269
- DOI: 10.1083/jcb.201012093
Defective nucleotide excision repair with normal centrosome structures and functions in the absence of all vertebrate centrins
Abstract
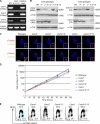
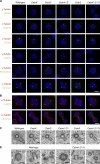
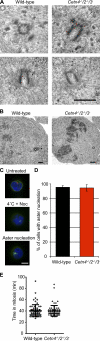
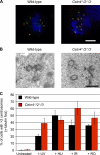
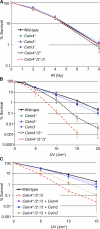
The principal microtubule-organizing center in animal cells, the centrosome, contains centrin, a small, conserved calcium-binding protein unique to eukaryotes. Several centrin isoforms exist and have been implicated in various cellular processes including nuclear export and deoxyribonucleic acid (DNA) repair. Although centrins are required for centriole/basal body duplication in lower eukaryotes, centrin functions in vertebrate centrosome duplication are less clear. To define these roles, we used gene targeting in the hyperrecombinogenic chicken DT40 cell line to delete all three centrin genes in individual clones. Unexpectedly, centrin-deficient cells underwent normal cellular division with no detectable cell cycle defects. Light and electron microscopy analyses revealed no significant difference in centrosome composition or ultrastructure. However, centrin deficiency made DT40 cells highly sensitive to ultraviolet (UV) irradiation, with Cetn3 deficiency exacerbating the sensitivity of Cetn4/Cetn2 double mutants. DNA damage checkpoints were intact, but repair of UV-induced DNA damage was delayed in centrin nulls. These data demonstrate a role for vertebrate centrin in nucleotide excision repair.
Figures
References
-
- Acu I.D., Liu T., Suino-Powell K., Mooney S.M., D’Assoro A.B., Rowland N., Muotri A.R., Correa R.G., Niu Y., Kumar R., Salisbury J.L. 2010. Coordination of centrosome homeostasis and DNA repair is intact in MCF-7 and disrupted in MDA-MB 231 breast cancer cells. Cancer Res. 70:3320–3328 10.1158/0008-5472.CAN-09-3800 - DOI - PMC - PubMed
-
- Araki M., Masutani C., Takemura M., Uchida A., Sugasawa K., Kondoh J., Ohkuma Y., Hanaoka F. 2001. Centrosome protein centrin 2/caltractin 1 is part of the xeroderma pigmentosum group C complex that initiates global genome nucleotide excision repair. J. Biol. Chem. 276:18665–18672 10.1074/jbc.M100855200 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

