Epidemiology and infectivity of Plasmodium falciparum and Plasmodium vivax gametocytes in relation to malaria control and elimination
- PMID: 21482730
- PMCID: PMC3122489
- DOI: 10.1128/CMR.00051-10
Epidemiology and infectivity of Plasmodium falciparum and Plasmodium vivax gametocytes in relation to malaria control and elimination
Abstract
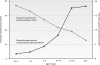
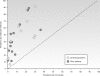
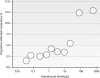
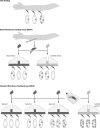
Malaria remains a major cause of morbidity and mortality in the tropics, with Plasmodium falciparum responsible for the majority of the disease burden and P. vivax being the geographically most widely distributed cause of malaria. Gametocytes are the sexual-stage parasites that infect Anopheles mosquitoes and mediate the onward transmission of the disease. Gametocytes are poorly studied despite this crucial role, but with a recent resurgence of interest in malaria elimination, the study of gametocytes is in vogue. This review highlights the current state of knowledge with regard to the development and longevity of P. falciparum and P. vivax gametocytes in the human host and the factors influencing their distribution within endemic populations. The evidence for immune responses, antimalarial drugs, and drug resistance influencing infectiousness to mosquitoes is reviewed. We discuss how the application of molecular techniques has led to the identification of submicroscopic gametocyte carriage and to a reassessment of the human infectious reservoir. These components are drawn together to show how control measures that aim to reduce malaria transmission, such as mass drug administration and a transmission-blocking vaccine, might better be deployed.
Figures




References
-
- Abdel-Wahab A., et al. 2002. Dynamics of gametocytes among Plasmodium falciparum clones in natural infections in an area of highly seasonal transmission. J. Infect. Dis. 185:1838–1842 - PubMed
-
- Abu-Raddad L. J., Patnaik P., Kublin J. G. 2006. Dual infection with HIV and malaria fuels the spread of both diseases in sub-Saharan Africa. Science 314:1603–1606 - PubMed
-
- Adjuik M., et al. 2004. Artesunate combinations for treatment of malaria: meta-analysis. Lancet 363:9–17 - PubMed
-
- Akim N. I., et al. 2000. Dynamics of P. falciparum gametocytemia in symptomatic patients in an area of intense perennial transmission in Tanzania. Am. J. Trop. Med. Hyg. 63:199–203 - PubMed
-
- Alano P. 2007. Plasmodium falciparum gametocytes: still many secrets of a hidden life. Mol. Microbiol. 66:291–302 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

