Mucormycosis caused by unusual mucormycetes, non-Rhizopus, -Mucor, and -Lichtheimia species
- PMID: 21482731
- PMCID: PMC3122490
- DOI: 10.1128/CMR.00056-10
Mucormycosis caused by unusual mucormycetes, non-Rhizopus, -Mucor, and -Lichtheimia species
Abstract
Rhizopus, Mucor, and Lichtheimia (formerly Absidia) species are the most common members of the order Mucorales that cause mucormycosis, accounting for 70 to 80% of all cases. In contrast, Cunninghamella, Apophysomyces, Saksenaea, Rhizomucor, Cokeromyces, Actinomucor, and Syncephalastrum species individually are responsible for fewer than 1 to 5% of reported cases of mucormycosis. In this review, we provide an overview of the epidemiology, clinical manifestations, diagnosis of, treatment of, and prognosis for unusual Mucormycetes infections (non-Rhizopus, -Mucor, and -Lichtheimia species). The infections caused by these less frequent members of the order Mucorales frequently differ in their epidemiology, geographic distribution, and disease manifestations. Cunninghamella bertholletiae and Rhizomucor pusillus affect primarily immunocompromised hosts, mostly resulting from spore inhalation, causing pulmonary and disseminated infections with high mortality rates. R. pusillus infections are nosocomial or health care related in a large proportion of cases. While Apophysomyces elegans and Saksenaea vasiformis are occasionally responsible for infections in immunocompromised individuals, most cases are encountered in immunocompetent individuals as a result of trauma, leading to soft tissue infections with relatively low mortality rates. Increased knowledge of the epidemiology and clinical presentations of these unusual Mucormycetes infections may improve early diagnosis and treatment.
Figures

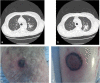


References
-
- Aarset H., Aasarod K., Bergan U., Angelsen A. 2001. Acute renal infarction in a woman with slight asthma. Nephrol. Dial. Transplant. 16:1711–1712 - PubMed
-
- Abdel-Hafez S. I., Moubasher A. H., Shoreit A. A., Ismail M. A. 1990. Fungal flora associated with combine harvester wheat and sorghum dusts from Egypt. J. Basic Microbiol. 30:467–479 - PubMed
-
- Ajello L., Dean D. F., Irwin R. S. 1976. The zygomycete Saksenaea vasiformis as a pathogen of humans with a critical review of the etiology of zygomycosis. Mycologia 68:52–62 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

