Synthesis and biosynthetic trafficking of membrane lipids
- PMID: 21482741
- PMCID: PMC3140686
- DOI: 10.1101/cshperspect.a004713
Synthesis and biosynthetic trafficking of membrane lipids
Abstract
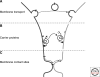
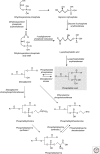
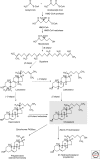
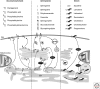
Eukaryotic cells can synthesize thousands of different lipid molecules that are incorporated into their membranes. This involves the activity of hundreds of enzymes with the task of creating lipid diversity. In addition, there are several, typically redundant, mechanisms to transport lipids from their site of synthesis to other cellular membranes. Biosynthetic lipid transport helps to ensure that each cellular compartment will have its characteristic lipid composition that supports the functions of the associated proteins. In this article, we provide an overview of the biosynthesis of the major lipid constituents of cell membranes, that is, glycerophospholipids, sphingolipids, and sterols, and discuss the mechanisms by which these newly synthesized lipids are delivered to their target membranes.
Figures

References
-
- Achleitner G, Gaigg B, Krasser A, Kainersdorfer E, Kohlwein SD, Perktold A, Zellnig G, Daum G 1999. Association between the endoplasmic reticulum and mitochondria of yeast facilitates interorganelle transport of phospholipids through membrane contact. Eur J Biochem 264: 545–553 - PubMed
-
- Achleitner G, Zweytick D, Trotter PJ, Voelker DR, Daum G 1995. Synthesis and intracellular transport of aminoglycerophospholipids in permeabilized cells of the yeast, Saccharomyces cerevisiae. J Biol Chem 270: 29836–29842 - PubMed
-
- Arakane F, Sugawara T, Nishino H, Liu Z, Holt JA, Pain D, Stocco DM, Miller WL, Strauss JF III 1996. Steroidogenic acute regulatory protein (StAR) retains activity in the absence of its mitochondrial import sequence: Implications for the mechanism of StAR action. Proc Natl Acad Sci 93: 13731–13736 - PMC - PubMed
-
- Baumann NA, Sullivan DP, Ohvo-Rekila H, Simonot C, Potteka A, Klaassen Z, Beh CT, Menon AK 2005. Transport of newly synthesized sterol to the sterol-enriched plasma membrane occurs via nonvesicular equilibration. Biochemistry 44: 5816–5826 - PubMed
-
- Ben-David O, Futerman AH 2010. The role of the ceramide acyl chain length in neurodegeneration: Involvement of ceramide synthases. Neuromolecular Med 12: 341–350 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
