L-fucose utilization provides Campylobacter jejuni with a competitive advantage
- PMID: 21482772
- PMCID: PMC3084102
- DOI: 10.1073/pnas.1014125108
L-fucose utilization provides Campylobacter jejuni with a competitive advantage
Abstract
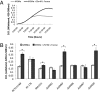
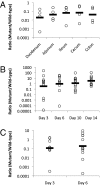
Campylobacter jejuni is a prevalent gastrointestinal pathogen in humans and a common commensal of poultry. When colonizing its hosts, C. jejuni comes into contact with intestinal carbohydrates, including L-fucose, released from mucin glycoproteins. Several strains of C. jejuni possess a genomic island (cj0480c-cj0490) that is up-regulated in the presence of both L-fucose and mucin and allows for the utilization of L-fucose as a substrate for growth. Strains possessing this genomic island show increased growth in the presence of L-fucose and mutation of cj0481, cj0486, and cj0487 results in the loss of the ability to grow on this substrate. Furthermore, mutants in the putative fucose permease (cj0486) are deficient in fucose uptake and demonstrate a competitive disadvantage when colonizing the piglet model of human disease, which is not paralleled in the colonization of poultry. This identifies a previously unrecorded metabolic pathway in select strains of C. jejuni associated with a virulent lifestyle.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

