Orthograde dihydropyridine receptor signal regulates ryanodine receptor passive leak
- PMID: 21482776
- PMCID: PMC3084091
- DOI: 10.1073/pnas.1018380108
Orthograde dihydropyridine receptor signal regulates ryanodine receptor passive leak
Abstract
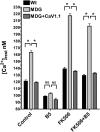
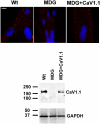
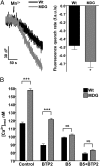
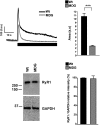
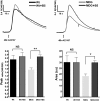
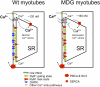
The skeletal muscle dihydropyridine receptor (DHPR) and ryanodine receptor (RyR1) are known to engage a form of conformation coupling essential for muscle contraction in response to depolarization, referred to as excitation-contraction coupling. Here we use WT and Ca(V)1.1 null (dysgenic) myotubes to provide evidence for an unexplored RyR1-DHPR interaction that regulates the transition of the RyR1 between gating and leak states. Using double-barreled Ca(2+)-selective microelectrodes, we demonstrate that the lack of Ca(V)1.1 expression was associated with an increased myoplasmic resting [Ca(2+)] ([Ca(2+)](rest)), increased resting sarcolemmal Ca(2+) entry, and decreased sarcoplasmic reticulum (SR) Ca(2+) loading. Pharmacological control of the RyR1 leak state, using bastadin 5, reverted the three parameters to WT levels. The fact that Ca(2+) sparks are not more frequent in dysgenic than in WT myotubes adds support to the hypothesis that the leak state is a conformation distinct from gating RyR1s. We conclude from these data that this orthograde DHPR-to-RyR1 signal inhibits the transition of gated RyR1s into the leak state. Further, it suggests that the DHPR-uncoupled RyR1 population in WT muscle has a higher propensity to be in the leak conformation. RyR1 leak functions are to keep [Ca(2+)](rest) and the SR Ca(2+) content in the physiological range and thus maintain normal intracellular Ca(2+) homeostasis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
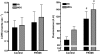
References
-
- Perez CF, López JR, Allen PD. Expression levels of RyR1 and RyR3 control resting free Ca2+ in skeletal muscle. Am J Physiol Cell Physiol. 2005;288:C640–C649. - PubMed
-
- Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: Dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. - PubMed
-
- Turner PR, Westwood T, Regen CM, Steinhardt RA. Increased protein degradation results from elevated free calcium levels found in muscle from mdx mice. Nature. 1988;335:735–738. - PubMed
-
- Yang T, et al. Elevated resting [Ca(2+)](i) in myotubes expressing malignant hyperthermia RyR1 cDNAs is partially restored by modulation of passive calcium leak from the SR. Am J Physiol Cell Physiol. 2007;292:C1591–C1598. - PubMed
-
- Lopez JR, Gerardi A, Lopez MJ, Allen PD. Effects of dantrolene on myoplasmic free [Ca2+] measured in vivo in patients susceptible to malignant hyperthermia. Anesthesiology. 1992;76:711–719. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

